Chemistry 9 Solved Paper 2019 Federal Board
Federal Board, Class 9 Chemistry Solved Past Paper 2019 is given below. The paper is solved according to the reduced syllabus for the annual examination 2021. Chemistry 9 Solved Paper 2019 is solved using the recommended textbook.
Chemistry 9 Solved Paper 2019
Section A
Q1. MCQs
Chemistry 9 Solved Paper 2019
Section B
Q2. Attempt any ELEVEN parts. The answer of each part should not exceed 3 to 4 lines.
(i) Define molecular mass and formula mass. Also write an example of each.
Ans: Molecular mass: The molecular mass of a compound is calculated by the sum of the atomic masses of all the atoms present in molecules.
Example: Molecules mass of H2O is 18.01 amu
Formula mass: The formula mass of a substance is the sum of atomic masses of all the atoms present in the formula unit of an ionic compound.
Example: Formula mass of NaCl is 58.5 amu
(ii) The atomic mass of copper metal is 63.6 amu. Calculate the mass of 3.5 moles of copper.
Solution: Number of moles of Cu = 3.5 moles
The atomic mass of Cu = 63.6 amu
Mass of 3.5 moles of Cu =?
Formula: Mass = number of Moles x Atomic mass
Mass = 3.5 x 63.6
Mass = 222.6 g
(iii) Write the group and period number of Phosphorus 3115P and Boron 10.65B from their valence shell electronic configuration
Ans. The electronic configuration of P = 1s2, 2s2, 2p6, 3s2, 3p3
Valence shell has configuration = 3s2, 3p3
Period number = 3
Group number = 2 + 3 = 5
Thus, P is present in the 3rd Period of Group VA.
The electronic configuration of B = 1s2, 2s2, 2p1
Valence shell has configuration = 2s2, 2p1
Period number = 2
Group number = 2 + 1 = 3
Therefore, B is present in the 2nd Period of Group IIIA.
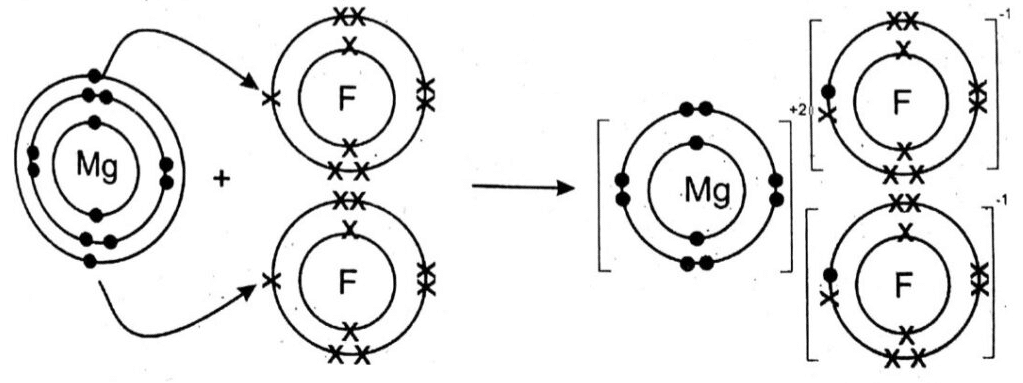
(iv) Briefly explain the formation of ionic bond between Magnesium and Fluorine with the help of diagram.
Ans: Mg belongs to II-A group of the periodic table. Its atomic number is 12. It is a metal as it contains 2 free electrons in its outermost shell due to which it loses these electrons and form Mg+2 ion.
F belongs to VII-A group of the periodic table. Its atomic number is 9. It is a non-metal as it contains 7 electrons in its outermost shell. Thus in order to complete its octet F atom gain 1 electron and form F ions.
For every Mg+2 ion, we need 2F – ions. Therefore, MgF2 is an ionic compound.
Therefore, MgF2 is an ionic compound.
(v) Briefly explain the conductance of electricity by molten sodium chloride without diagrams.
Excluded from Syllabus
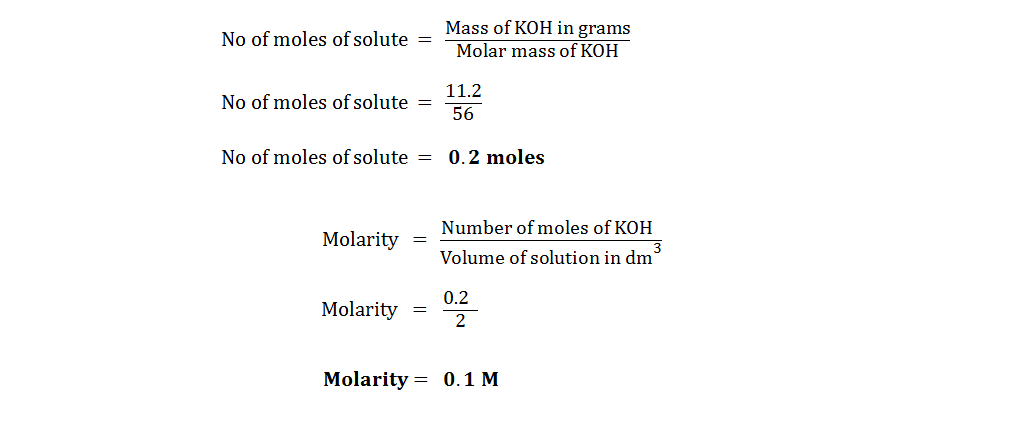
(vi) A chemist dissolves 11.2 g of KOH (molar mass 56) in two dm3 of solution. Find its morality.
Solution: Mass of KOH = 11.2 g
Molar mass of KOH = 56 g/mol
Volume of solution = 2 dm3
Molarity (M) = ?
(vii) Compare solution and suspension in three points.
Ans:
| Solution | Suspension |
| i. A homogenous mixture of two of more than two substances, in which the solute particles cannot be seen with naked eye, is known as solution or true solution. | i. In colloidal solution, the solute particles do not homogenize with solvent. These particles are a little bit bigger than the true solution but cannot be seen with the naked eye. |
| ii. The particles of true solution pass through the filter paper leaving no precipitate. | ii. The particles of colloidal solution do not settle down and pass through the filter paper. |
| iii. It can’t scatter light. | iii. It can scatter light. |
| iv. Example: NaCI in water | iv. Example: Jams, Halwa |
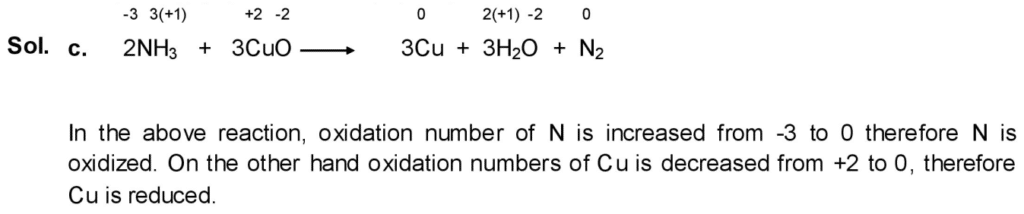
(viii) Which substance is reduced and which one is oxidized in each of the following reactions:
a. N2 + 3H2 → 2NH3
b. Cl2 + H2S → 2HCI + S
c. 2NH3 + 3CuO → 3Cu + 3H2O + N2


(ix) Explain Zinc plating with the help of chemical equations.
Ans: Zinc plating on steel is done by using zinc metal as an anode. A solution of potassium zinc cyanide K2[Zn(CN)4] containing little sodium cyanide. The steel object is made the cathode. During the electrolysis zinc at the anode enters the solution as Zn+2 ions, which are deposited at the cathode. The electrolyte ionizes as follows.
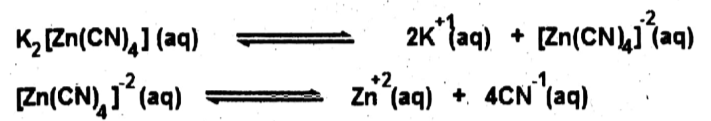
Redox reactions: Following reactions occur at the electrodes:
At anode:
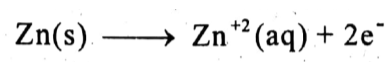
At cathode:
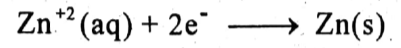
(x) Noble metals are inert. Elaborate this statement.
Ans: The chemistry of metals is characterized by their ability to lose electrons. The metals that are relatively difficult to oxidize are called Noble metals. All active metals react with Ha but noble metals don’t. Gold and platinum only react with aqua regia.
Examples; Gold, Copper etc.
(xi) Write the electronic configuration of the following by using 1s, 2s …… form.
Ans. (a) The atomic number of Cl = 17
The electronic configuration of Cl = 1s2, 2s2, 2p6, 3s2, 3p5
(b) The atomic number of Si = 14
The electronic configuration of Si = 1s2, 2s2, 2p6, 3s2, 3p2
(c) The atomic number of Ne = 10
The electronic configuration of Si = 1s2, 2s2, 2p6
Chemistry 9 Solved Paper 2019
Section C
Note: Attempt any TWO questions. All questions carry equal marks.
Q3 a. A molecule has two nitrogen and four hydrogen atoms. If the symbols of nitrogen and hydrogen are 11N and 1H respectively then write its empirical formula and determine its molar mass.
Solution: Molecular formula = N2H4 Empirical formula = NH2
Molar mass of N2H4= 2 x 14 +1 x 4 = 28 + 4 = 32 g/mole
Q3 b. Define ionization energy and atomic size. Also write their trends in periodic table.
Ans: Ionization energy:
The minimum amount of energy required to remove an electron from the outermost shell of an isolated gaseous atom is known as the first ionization energy. The unit of ionization energy is Kj mole-1.
The trend of Ionization Energy in the group:
Ionization energy decreases from top to bottom in a group because of an increase in atomic radius and shielding effect.
The trend of Ionization Energy in period:
Ionization energy increases from left to right in a period because of an increase in nuclear charge.
Atomic size:
The size of an atom is the average distance between the nucleus of an atom and its outermost electronic shell.
The trend of Atomic size or atomic radius in the group:
The atomic radius of an atom increases down the group because of an increase in the number of shells and the shielding effect.
The trend of Atomic size or atomic radius in period:
The atomic radius of an atom decreases from left to right in a period due to an increase in effective nuclear charge.
Q4 a. Define octet rule. Using this rule, show the formation of carbon dioxide molecule with dot and cross model.
Ans: Octet rule:
The tendency of atoms to acquire eight electron configurations in their valence shell, when bonding, is known as the octet rule.
Formation of Carbon Dioxide molecule:
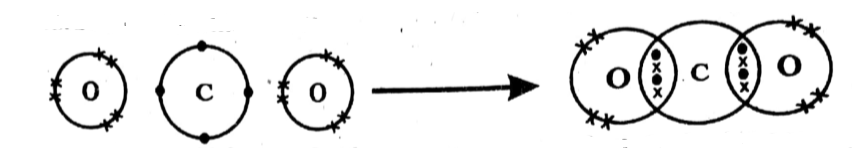
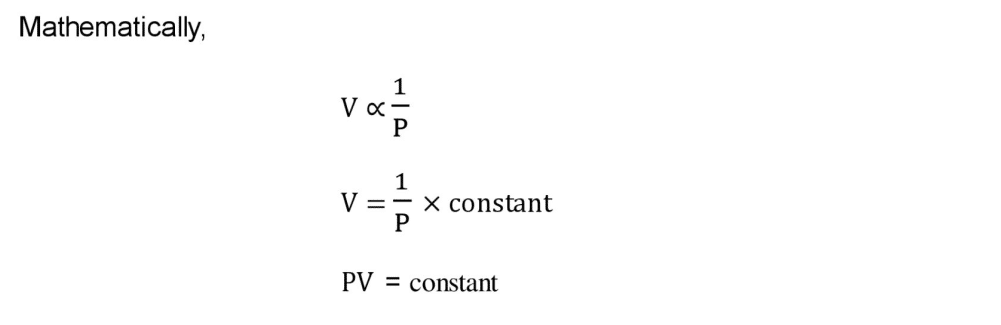
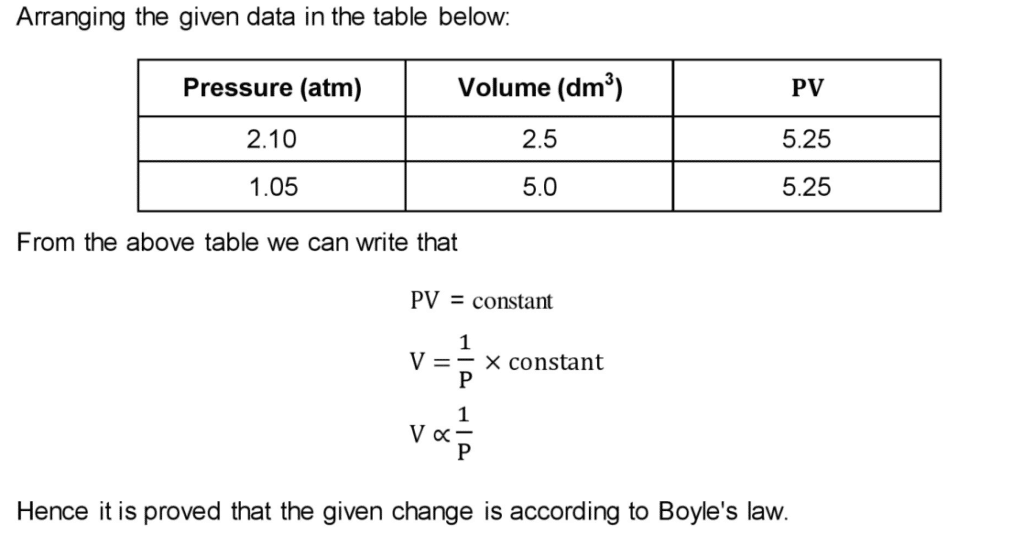
Q4 b. Define Boyle’s law. The volume of ethane gas is 2.5 dm3 at 2.10 atm pressure. When its pressure is reduced to 1.05 atm its volume becomes 5.0 dm3. Explain this change using Boyle’s law.
Ans: Boyle’s Law
“The volume of a given mass of a gas directly is proportional to pressure applied keeping the temperature constant”.
Q5. Define solubility. Explain the effect of temperature on the solubility of ionic solids and gases.
Ans: Solubility:
The amount of solute in grams, which can dissolve in 100 g of the solvent to give a saturated solution at a particular temperature, is known as the solubility of a solute.
Effect of temperature on solubility of different ionic compounds:
Solubility of some substances increases with the increase in temperature e.g. KCI, KBr, NaNO3, and NH4CI.
If we add 34.7 g of KCI to 100 g of water at 20 °C, it will dissolve. If we add more than 34.7 g of KCI at 20 °C, it will not dissolve. However, if we increase temperature it will readily dissolve. Keep on adding more KCI and increase temperature. We will see that by increasing temperature the solubility goes on increasing until 56.7 g of KCI is dissolved in 100 g of water at 100°C. Now cool the solution till the temperature drops to 20°C. At that temperature, 34.7 g of KCI dissolves and 22 g are crystallized out due to a decrease in temperature and results in a decrease in the solubility of KCI.
Solubility of some substances decreases with the increases in temperature e.g. Na2SO4.
The solubility of some substances is least affected by a change in temperature e.g. NaCI.
Effect of temperature on solubility of gases:
The solubility of gases e.g. air in water decreases with the increase in temperature because when water is heated, the bubbles of air come out of the water before the boiling of water.