Chemistry 9 Solved Model Paper 2020 Federal Board
Federal Board, Class 9 Chemistry Solved Model Paper 2020 is given below. The paper is solved according to the reduced syllabus for the annual examination 2021.
Class 9 Chemistry Solved Model Paper 2020
Section A
Q1. MCQs
Chemistry 9 Solved Model Paper 2020
Section B
Q2. Attempt any SIX parts from the following. All parts carry equal marks.
i. Why one mole of Oxygen gas and one mole of oxygen atoms have different masses?
Ans. Oxygen gas occurs as a diatomic molecule, therefore, the mass of one mole of Oxygen gas (O2) is equal to its molecular mass in grams i.e. 2×16=32 g. whereas the mass of one mole of oxygen atoms (O) is equal to its atomic mass in grams i.e. 16 g.
ii. Give three differences between atom and molecule.
Ans: Differences between atom and molecule:
| Atom | Molecule |
| Atom is the smallest particle of an element. | The molecule is the smallest particle of a substance that can exist independently. |
| Atom cannot exist in a free state. Atom Comprise of Nucleus and electrons. | Molecule Comprise of Two or more, identical or different atoms, bonded chemically. |
| Atom is electrically neutral. The shape of an atom is spherical Examples: Oxygen – O Phosphorus – P Sulphur – S Hydrogen – H | Whereas the molecules can be linear, angular or rectangular in shape. Examples: Oxygen – O2 Phosphorus – P4 Sulphur – S8 Water – H2O |
iii. Write atomic number and names of elements X, Y, Z, where:
X = 1s2 2s2 2p4, Y = 1s2 2s2 2p6 3s1, Z = 1s22s22p6 3s2 3p3
Ans. X= 1s2 2s2 2p4 Total number of electrons = 8 Therefore atomic number = 8 Name of element is Oxygen (O). Y= 1s2 2s2 2p6 3s1 Total number of electrons = 11 Therefore atomic number = 11 Name of element is Sodium (Na). Z= 1s2 2s2 2p6 3s2 3p3 Total number of electrons = 15 Therefore atomic number = 15 Name of element is Phosphorus (P).
iv a. Give notations for sub-shells of M-shell.
Ans: Notation for M shell is n =3 So M shell has 3 sub-shells called 3s , 3p and 3d.
iv b. Arrange the sub-shells of M-shell in order of increasing energy.
Ans: The increasing order of energy of the sub-shells belonging to M- shell is given; 3s < 3p< 3d
v. Determine the demarcation of the periodic table into ‘s block’ and ‘p block’.
Ans. On the basis of the outermost valence sub-shell, elements in the periodic table can also be classified into four blocks i.e. s, p, d, and f blocks.
Elements of Group IA and Group IIA contain their valence electrons in s sub-shell. Therefore, these elements are called s block elements.
The elements of Group IIIA to VIIIA (except He) are known as p block elements because their valence electrons lie in the p sub-shell.
vi. The elements present in a group of the periodic table have similar chemical properties. Give reason and a suitable example.
Ans. Reason: Chemical properties depend on the valence shell electronic configuration. Because all the elements of a particular group have similar valence shell electronic configuration, they possess similar chemical characteristics.
Examples: Group IA elements have similar chemical properties and are called Alkali metals, similarly, group IIA elements are called Alkaline Earth metals.
vii. What type of bond exists between two non-metallic atoms of different elements? Give an elaborative example.
Ans: Polar covalent bond exists between two non-metallic atoms of different elements.
The covalent bond formed between two unlike atoms which differ in their electronegativity is said to be a polar covalent bond.
Explanation: When two different atoms share electron pairs, both the atoms exert different forces on the shared electron pair. More electronegative atom pulls shared electrons PEs with greater force than the other. So more electronegative atom partially draws electron density toward itself. This makes it partially negatively charged and another atom partially positively charged. Such a covalent bond is called a polar covalent bond.
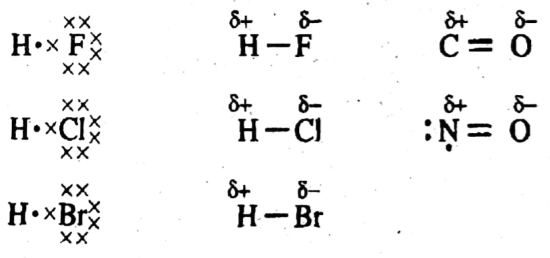
viii. With the help of a suitable diagram show the conductance of electricity through molten NaCl. Also, write the reactions at Anode and Cathode.
Excluded from Syllabus
Chemistry 9 Solved Model Paper 2020
Section C
Q3. Attempt any FIVE parts from the following. All parts carry equal marks.
i. How does temperature affect the vapour pressure of a liquid?
Ans: The vapour pressure of liquid increases as the temperature increases. The molecules in the liquid are more energetic at higher temperatures, and more molecules can escape froth the liquid phase into the gaseous phase.
Vapour pressure of a liquid ∝ Temperature
ii. Give three differences between heavy water and ordinary water.
Excluded from Syllabus
iii. What will happen, when a crystal of sodium Sulphate is added to
a. An unsaturated solution of sodium sulphate
Ans: When a crystal of sodium sulphate is added to an unsaturated solution of sodium sulphate, it will dissolve in it.
b. Saturated solution of sodium sulphate
Ans: When a crystal of sodium sulphate is added to a saturated solution of sodium sulphate, it will drop to the bottom without dissolving.
c. Supersaturated solution of sodium sulphate
Ans: When a crystal of sodium sulphate is added to a supersaturated solution of sodium sulphate, crystallization will start. When crystallization will finish, we will get a saturated solution in presence of its sodium sulphate crystals.
iv. Write three different units of pressure? Give their relation with one another.
Ans: The unit of pressure in the SI system is the pascal (Pa), defined as a force of one Newton per square meter. The conversion between atm, Pa, and the torr is as follows:
1 atm = 101325 Pa = 760 torr
1 atm = 760mm Hg = 760 torr
v. Aluminium has a higher tendency to oxidize than iron but still it is considered as safe metal. Justify your answer with reason.
Ans: The corrosion resistance of aluminum is dependent upon a protective oxide film.
This is because a tough layer of insoluble aluminum oxide (Al2O3) forms on its surface when aluminum metal is exposed to air. This layer firmly adheres to the metal and serves to protect the underlying aluminum layers from further corrosion.
On the other hand, the insoluble layer of rust, Fe2O3xH2O that forms on the surface of iron is too porous to protect the underlying metal. This layer flakes away and exposes metal for further corrosion.
vi. Find the oxidation state of underlined atoms.
a) S2Cl2 b) Zn(OH)2 c) OF2
Sol. a) S2Cl2
2(x) + 2(-1) = 0
2x – 2 = 0
2x = +2
x = +2/2
x = +1
b) Zn(OH)2
x + 2(-1) = 0
x – 2 = 0
x = + 2
c) OF2
x + 2(-1) = 0
x – 2 = 0
x = + 2
vii. Oxidizing power of Fluorine is the highest among other halogens. Write any two chemical reactions to show this property of Fluorine.
Ans: Oxidizing power of F2 is the highest and that of I2 is the lowest. Due to the relative strength of an oxidizing agent, it is possible for a free halogen to oxidize the ion of halogen next to it in the group. This means F2 can oxidize all the halide ions to free halogen.
For example,
F2 (g) + 2KCl(aq) —> 2KF(aq) + Cl2(g)
F2 (g) + 2KBr(aq) —> 2KF(aq) + Br2(l)
F2(g) + 2NaI(aq) —> 2NaF(aq) + I2(s)
Similarly, Cl2 can oxidize Br– and I– ions. But I2 cannot oxidize any halide ion.
Chemistry 9 Solved Model Paper 2020
Section D
Note: Attempt any TWO questions. All questions carry equal marks.
Q4 a. How many moles and atoms are there in 60 grams of Calcium?
Ans: Mass in gram = 60 g
Atomic mass of Calcium (Ca) = 40 amu
Molar mass of Calcium (Ca) = 40 g
Avogadro’s number = NA = 6.022 x 1023
Number of moles =?
Number of atoms =?
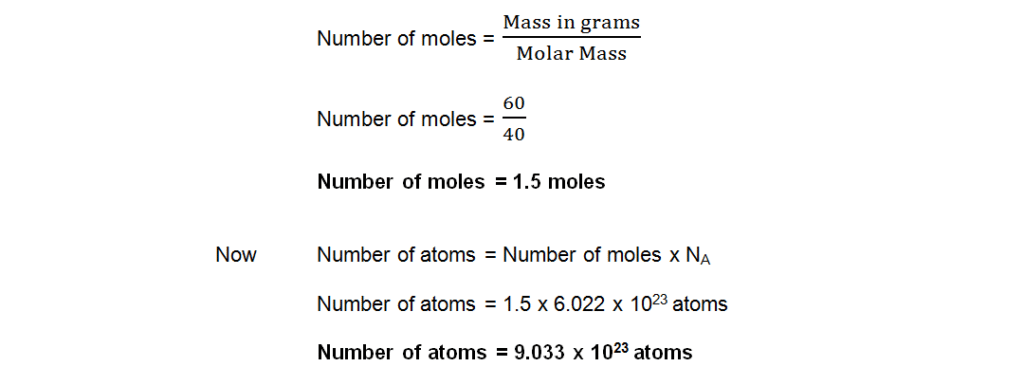
Q4 b. What were the defects in Rutherford’s atomic model and how were these removed by Bohr?
Ans: Defects In Rutherford’s atomic model:
Rutherford’s model of an atom resembles our solar system. It has the following defects:
1. Classical physics suggests that electron being charged particles will emit energy continuously while revolving around the nucleus. Thus the orbit of the revolving electron becomes smaller and smaller until it would fall into the nucleus. This would collapse the atomic structure.
2. If a revolving electron emits energy continuously it should form a continuous spectrum for an atom but a line spectrum is obtained.
Correction of defects in Rutherford’s atomic model by Bohr:
Bohr removed the defects of Rutherford’s atomic theory by using Quantum theory.
1. He explained that electrons do not emit energy continuously but they emit or release energy when they move from one orbit to another.
2. He explained the reason for the line spectrum observed instead of a continuous spectrum.
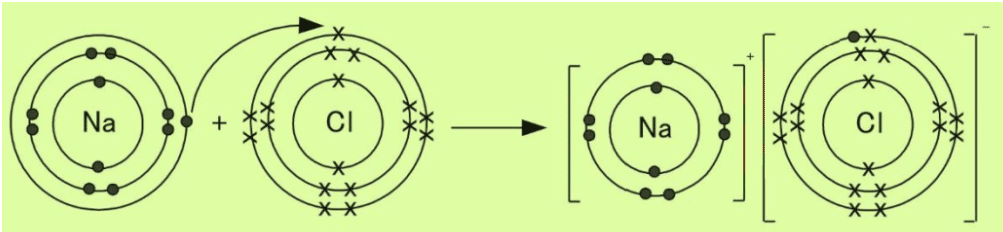
Q.5 a. State and explain ionic bond and its formation between sodium and chlorine atoms to form sodium chloride.
Ans. Ionic Bond
The forces of attraction that bind oppositely charged ions are called ionic bonds.
The ionic bond is formed between sodium and chlorine. Sodium (Na) is metal and (Chlorine) Cl is non-metal.
Metal atom tends to lose electrons and non-metal atom tends to gain electrons to acquire electronic configuration of the nearest noble gas. Since the Na atom has one electron in the outermost shell, it losses one electron to form the Na+ ion. Since the Cl atom has seven electrons in the outermost shell, it needs one electron to complete the octet. So it gains one electron to form a Cl– ion. For every Na+ ion, we need one Cl– ion.
Q5 b. State Charles’s law. If a balloon occupies 885 cm3 volume at 20°C and 794 cm3 at -10°C, Prove that this data is according to Charles’s law.
Ans: Charles’s law:
The law states that the volume of a given mass of a gas varies directly with the absolute temperature at constant pressure.
Mathematical form of Charles’s law:
V ∝ T
V = constant x T
V/T = constant
This relationship is known as Charles’s Law.
Table: Temperature-volume relationship of balloon
| Temperature (°C) | Volume (cm3) | Temperature (K) | V/T |
| 20 | 885 | 20 + 273 = 293 | 885/293 = 3 |
| -10 | 794 | -10 + 273 = 263 | 794/263 = 3 |
The ratio V/T = 3 is fairly constant. Thus the volume of the gas varies directly with the absolute temperature as stated by Charles’s law.
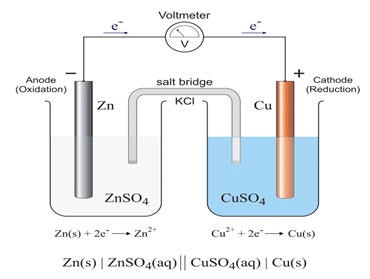
Q6 a. Sketch a Daniel cell, label cathode & anode, show the direction of flow of electrons, and write down chemical reactions occurring at cathode & anode.
Ans.
Q6 b. Copper, silver, and gold are called noble metals. Briefly explain their inertness toward chemical reactions.
Ans: Inertness of noble metals:
The chemistry of metals is characterized by their ability to lose electrons to form cations. Some metals such as copper, silver, gold, and platinum are relatively difficult to oxidize. Therefore, these metals are often called noble metals Gold and platinum exist mostly as free elements in nature. Copper and silver exist in both free and combined states.
All active metals react with HCI but noble metals do not react with HCI. Copper and silver react with strong oxidizing agents such as conc. HNO3 and HClO4.
Aqua regia:
Gold and platinum react only with aqua regia. Aqua regia is a mixture of 3 parts by volume of conc. HCI and one part by volume of conc. HNO3.

so much good
Azab hai azab
Usefull content