Chemistry 9 Solved Paper 2018 Federal Board
Federal Board, Class 9 Chemistry Solved Past Paper 2018 is given below. The paper is solved according to the reduced syllabus for the annual examination 2021. Chemistry 9 Solved Paper 2018 is solved using the recommended textbook.
Chemistry 9 Solved Paper 2018
Section A
Q1. MCQs
Class 9 Chemistry Solved Paper 2018
Section B
Q2. Attempt any ELEVEN parts. The answer of each part should not exceed 3 to 4 lines.
(i) Determine the molecular mass of:
a. (NH2)2CO b. NH4NO3 c. C6H12O6
Solution: a. Urea, (NH2)2CO
Molecular mass of (NH2)2CO = 2 x 14 + 2 x 2 + 12 + 16 = 28 + 4 + 12 + 16 = 60 amu
b. Ammonium nitrate, NH4NO3.
Molecular mass of NH4NO3 = 14 + 1 x 4 + 14 + 3 x 16 = 14 + 4 + 14 + 48 = 80 amu
c. Glucose, C6H12O6
Molecular mass of C6H12O6 = 12 x 6 +12 x 1 +16 x 6= 72 +12 +96 = 180 amu
(ii) Define atomic size. Interpret trends in atomic size across groups and periods.
Ans. Atomic size:
The size of an atom is the average distance between the nucleus of an atom and the outer electronic shell.
Trends in Groups:
The atomic size increases in any given main group as we move down the group of elements. This is because as
we move to the next lower element in the group, the atom has an additional shell of electrons.
Trends in Periods:
The atomic size decreases in any given period as we move across the period. This is because of the increasing positive charge on the nucleus caused by increasing atomic number from left to right in a period.
(iii) The boiling point of water on the top of Mount Everest is 70°C while in Murree it is 98°C. Give the reason.
Ans: We know that a liquid boils when its vapour pressure becomes equal to atmospheric pressure.
At sea level, the atmospheric pressure is 1 atm or 101.325 kPa so water boils at 100 °C. At Mount Everest at a height of about 8850m above sea level, the atmospheric pressure becomes about 34 kPa. Therefore at this low pressure, the water boils at 70 °C. In Murree the vapour pressure of water becomes equal to atmospheric pressure at 98 °C therefore water boils at 98°C in Murree.
(iv) Sketch out an electrolytic cell, label it and indicate the direction of flow of electrons.
Ans: Sketch of electrolytic cell
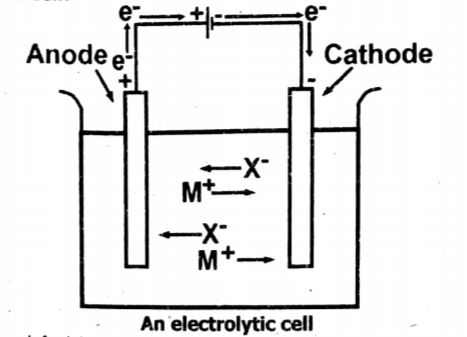
(v) Arrange the following in order of increasing acid strength: HCl , HI ,HBr and HF
Ans: The hydrogen halides dissolve in water to form hydrohalic acid e.g., hydrochloric acid HCI, hydrofluoric acid HF, etc. Except for HF, other hydrohalic are strong acids. The acidic strength increases in the following order:
HF < HCI < HBr < HI
(vi) Define oxidation and reduction in terms of loss and gain of electrons. Illustrate the concepts with the help of examples.
Ans: Oxidation: A process that involves the loss of electrons by an element is called oxidation.
Examples: (write any 1)
Na → Na+ + e–
Ca → Ca+2 + 2e–
Fe → Fe+2 + 2e–
Reduction: A process that involves the gain of electrons by a substance is called reduction.
Examples: (write any 1)
CI + e– → Cl–
O + 2e– → O-2
S + 2e– → S-2
(vii) Differentiate between Atom and ion with the help of suitable examples.
Ans: Difference between Atom and ion:
| Atom | Ion | |
| 1 | Atom is the smallest particle of an element that cannot exist in a free state. | An ion is a charged species formed from an atom or chemically bonded groups of atoms by adding or removing electrons. |
| 2 | It is electrically neutral. | On the other hand, an ion is a charged species. |
| 3 | Examples: Na, Ca, O and CI | Examples: Na+1 ,Ca+2 ,O-2 , Cl-1 |
(viii) How can oil tanks be prevented from corrosion? Support your answer with diagram and an example.
Ans: Prevention of oil tank from corrosion:
The oil tank that is to be protected from corrosion is made of the cathode and is connected to metals such as magnesium or aluminum. These metals are more active than iron, so they act as an anode and iron as a cathode.
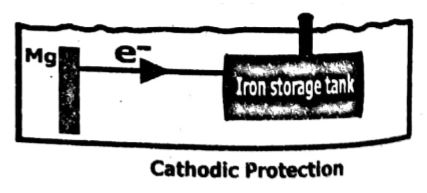
The more active metals themselves oxidize and save iron from corrosion.
Example: This method is employed to prevent iron and steel structures such as pipes, tanks, oil rigs, etc in the moist underground and marine environment.
Chemistry 9 Solved Paper 2018
Section C
Note: Attempt any TWO questions. All questions carry equal marks.
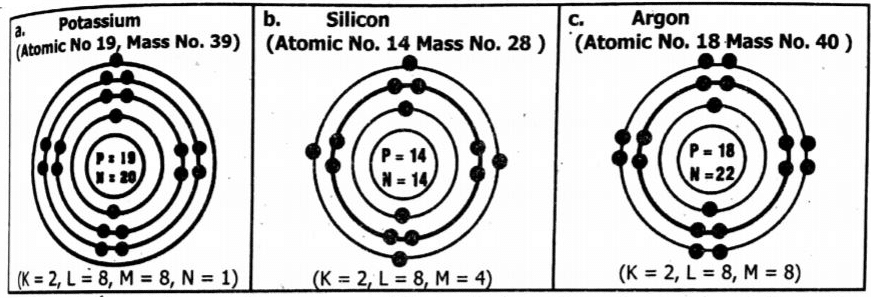
Q3 a. Make Bohr’s models for the following elements:
a. Potassium (3919 K) b. Silicon (2814Si) c. Argon (4018Ar)
Ans: Bohr’s models:
Q3 b. State postulates of Dalton’s atomic theory. What is the basic contribution of this theory for chemical science?
Ans: Dalton’s Atomic theory:
i. All elements are composed of tiny indivisible particles called atoms.
ii. Atoms of a particular element are identical. They have the same mass and the same volume.
iii. During chemical reaction atoms combine or separate or re-arrange. They combine in simple ratios.
iv. Atoms can neither be created nor destroyed.
Basic Contribution of Dalton’s Atomic theory:
Dalton’s atomic theory explained the law of chemical combinations.
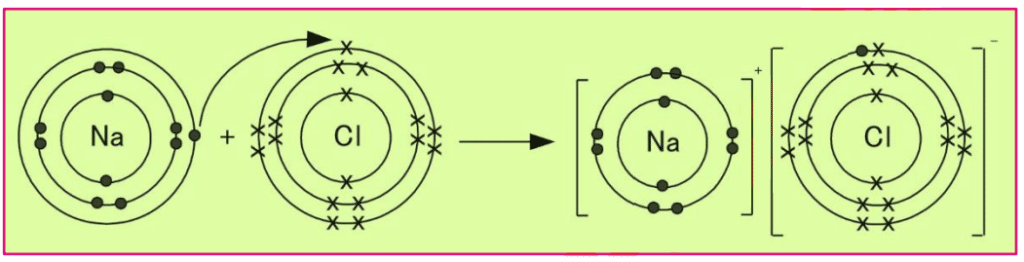
Q4 a. Define ionic bond, Use electron dot and cross model to write the equation for the formation of an ionic compound.
Ans: Ionic Bond:
The forces of attraction that bind oppositely charged ions are called ionic bonds.
Equation for the formation of an ionic compound:
Formation ionic bond in Sodium and Chlorine Na is metal and CI is non-metal.
Q4 b. What is hydrogen bonding? Explain hydrogen bonding in water by utilizing electron dot and cross structure.
Ans: Hydrogen bonding:
The interaction of a highly electron-deficient hydrogen and lone pair on a nearby highly electronegative atom such as N, O, or F is called a hydrogen bond. This phenomenon is called hydrogen bonding.
Hydrogen bonding in water molecules:
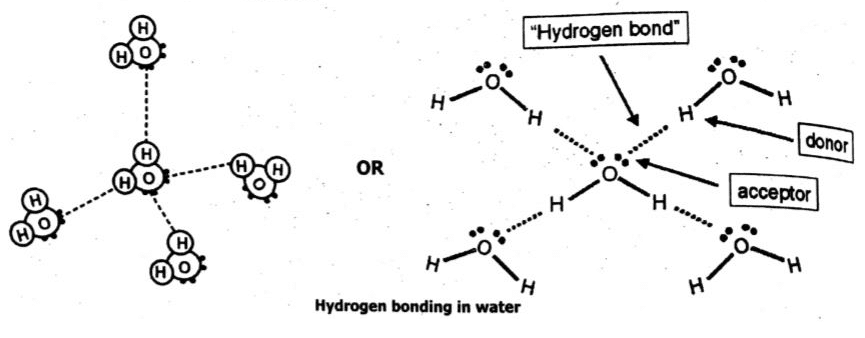
Q5 a. Whit is corrosion? Explain corrosion of Iron with the help of chemical equation.
Ans: Corrosion:
Corrosion is the process in which a metal reacts with oxygen and moisture in the atmosphere.
Corrosion of Iron:
Oxygen and water are necessary for iron to rust. A region of a metal surface that has relatively less moisture, acts as an anode.
Fe (s) → Fe+2 (aq) + 2e–
Another region on the surface of the metal that has relatively more moisture acts as a cathode. The electrons released in the oxidation process reduce atmospheric oxygen to hydroxyl ions.
O2 + 2H20 + 4e– → 40H–
The Fe+2 ions formed at the anodic regions flow to the cathodic regions through the moisture on the surface. Here Fe+2 ions further react with oxygen to form rust (Fe2O3.xH2O).
Q5 b. Sodium Chloride and Glucose both are soluble in water but the solubility of Sodium Chloride (NaCl) is greater than glucose. Why? Explain.
Ans: i. Glucose, whose molecule has many -O-H bonds, is very soluble in water
ii. Sodium chloride is an ionic compound and Glucose is a polar compound, due to the presence of ionic bonds in sodium chloride it is more soluble than Glucose.
Explanation:
Sodium chloride is an ionic compound. The negative end of water molecules is attracted to sodium ions and the positive end of water molecules is attracted to chloride ions. These attractive forces are strong enough to overcome the strong attractions that exist between ions in NaCl. Thus sodium chloride dissolves readily.


Good and thank you.