Chemistry 9 Solved Paper 2017 Federal Board FBISE
Federal Board, Class 9 Chemistry Solved Past Paper 2017 is given below. The paper is solved according to the reduced syllabus for the annual examination 2021. Chemistry 9 Paper 2017 is solved using the recommended textbook. For more solved past papers of class 9 visit our page.
Chemistry 9 Solved Paper 2017
Section A
Q1. MCQs
Chemistry 9 Solved Paper 2017
Section B
Q2. Attempt any ELEVEN parts. The answer of each part should not exceed 3 to 4 lines.
(i) Define Molecular mass. Find the molecular mass of C6H14 and C2H5OH if atomic masses of C=12, H=1 and O=16.
Ans. Molecular mass
Definition
“The sum of atomic masses of all the atoms present in one molecule of a molecular compound is called molecular mass.”
The term molecular mass is used for a molecular compound that is covalent in nature.
Molecular mass of C6H14 = 6 x 12 + 14 x 1
Molecular mass of C6H14 = 72 + 14
Molecular mass of C6H14 = 86 amu
Molecular mass of C2H5OH = 2 x 12 + 5 x 1 + 16 + 1
Molecular mass of C2H5OH = 24 + 5 + 16 + 1
Molecular mass of C2H5OH = 46 amu
(ii) Write any two differences between ions and free radicals. Also write an example of each.
Excluded from Syllabus
(iii) Write three conclusions drawn by Rutherford from his metal foil experiment.
Ans. Conclusions of Rutherford’s Experiment
Rutherford drew the following conclusions:
1. Since the majority of the α-particles passed through the foil undeflected, most of the space occupied by an atom must be empty.
2. The deflection of a few α-particles through angles greater than 90o shows that these
particles are deflected by electrostatic repulsion between the positively charged α-particles and the positively charged part of the atom.
3. Massive α-particles are not deflected by electrons.
(iv) Write the electronic configuration of the following elements by distributing electronics in their sub-shells (s, p, d, f):
a. Al b. Cl c. Ne
Ans. (a) Al = 1s2, 2s2, 2p6, 3s2, 3p1
(b) Cl = 1s2, 2s2, 2p6, 3s2, 3p5
(c) Ne = 1s2, 2s2, 2p6
(v) Define Atomic Size. Write the reason of increasing atomic size down the group and decreasing atomic size from left to right in the period of periodic table.
Ans. Atomic Size:
Definition:
“The size of an atom is the average distance between the nucleus of an atom and the outer electronic shell.”
Atomic size increases down the group
Reason:
The atom has an additional shell of electrons down the group which increases atomic radius. The size of an atom is determined by the size of its valence shell.
Atomic size decreases from left to right in the period
Reason:
One electron is added to the same valence shell and at the same time, the positive charge on the nucleus also increases by one. The attractive force of the nucleus from the valence shell electron increases therefore the atomic radius decreases.
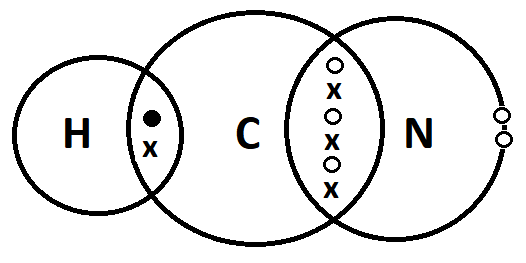
(vi) What is a covalent bond? Show the formation of a covalent bond between atoms in the following compounds with the help of cross and dot models: a. CO2 b. HCN (At. No C=6, N=7, O=8, H=1)
Ans. Covalent Bond
The type of chemical bond that is formed by the sharing of electrons between two atoms is called a covalent bond.
a. CO2
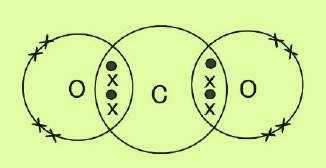
b. HCN
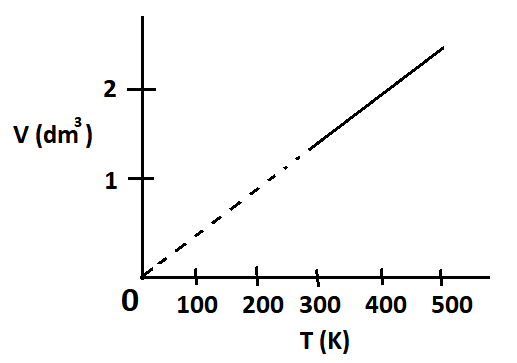
(vii) Define Charles’s Law. Derive relationship V/T = constant. Also show this relationship with the help of a graph.
Ans. Charles’s Law
This law states that the volume of a given mass of a gas is directly proportional to absolute temperature at constant pressure.
Mathematically,
V ∝ T
V = (constant) T
V / T = constant
(viii) Write any two differences between crystalline solids and amorphous solids. Also give one example of each.
Ans.
| Crystalline Solids | Amorphous Solids |
| 1. They are composed of orderly, repeating three-dimensional arrangements of particles. | 1. They lack an ordered arrangement of particles in them. |
| 2. They have well-defined shapes. | 2. They do not have well-defined shapes. |
| 3. They have sharp melting points. | 3. They do not have sharp melting points. |
| 4. Example: Sodium Chloride | 4. Example: Glass, Plastic |
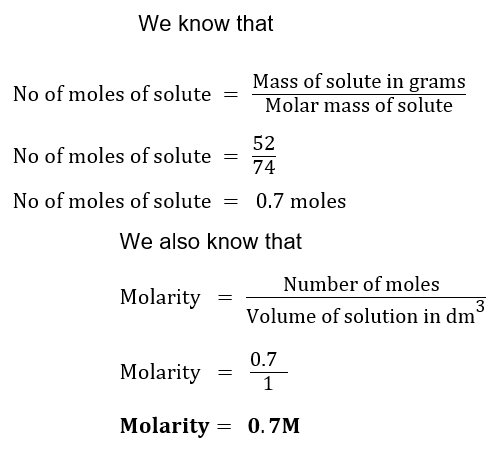
(ix) Find the molarity of 1000cm3 solution if it contains 52 grams of Ca(OH)2.
Sol. Mass of Ca (OH)2 = 52 g
Molecular mass of Ca (OH)2 = 40+17 x 2 = 74 amu
Molar mass of Ca (OH)2 = 74 g/mol
Volume of solution = 1000 cm3 = 1 dm3
Molarity =?
(x) Define oxidation number. Find the oxidation number of Boron in H3BO3 with the help of oxidation number rules.
Ans. Oxidation Number:
The oxidation number is defined as the number of charges an atom will have in a molecule or a compound.”
The elements that show an increase in oxidation number are oxidized.
The elements that show a decrease in oxidation number are reduced.
Oxidation number of Boron in H3BO3
3 (+1) + x + 3(-2) = 0
+3 + x – 6 = 0
x – 3 = 0
x = +3
(xi) Write a note on Zinc plating by giving chemical equations.
Ans. Zinc Plating:
Zinc plating on steel is done by using zinc metal as anode. A solution of potassium zinc cyanide K2[Zn(CN)4] containing little sodium cyanide is used as an electrolyte. The steel object is made the cathode. Sodium cyanide prevents the hydrolysis of the electrolyte.
Ionization of Electrolyte on Electrolysis
The electrolyte ionizes as follows:
K2[Zn(CN)4] (aq) ⇌ 2K+(aq) + [Zn(CN)4]-2(aq)
[Zn(CN)4]-2(aq) ⇌ Zn+2(aq) + 4CN-1(aq)
Reactions at Electrodes:
During the electrolysis zinc at the anode enters the solution as Zn+2 ions which are deposited at the cathode.
Reaction at Anode:
Zn → Zn+2 + 2e–
Reaction at Cathode:
Zn+2 + 2e– → Zn
(xi) a. What are noble metals? Write any two examples.
Ans. Some metals such as copper silver gold and platinum are relatively difficult to oxidize therefore these metals are often called noble metals, e.g., Platinum and Silver.
(xi)b. Write the composition of Aqua Regia.
Ans. Aqua Regia is a mixture of 3 parts by volume of conc. HCI and one part by volume of conc. HNO3.
(xiii) Choose and re-write two of the following reactions that are possible:
(a) F2 + 2KBr → 2KF + Br2
(b) I2 + 2KCl → 2KI + Cl2
(c) Cl2 + 2KI → 2KCl + I2
(d) Br2 + 2KCl → 2KBr + Cl2
Ans. Following two reactions are possible, only:
(a) F2 + 2KBr → 2KF + Br2
(c) Cl2 + 2KI → 2KCl + I2
(xiv) Define Unsaturated, Saturated and Supersaturated solutions.
Ans. Unsaturated Solution:
A solution that can dissolve more solute at a particular temperature is called an unsaturated solution.
Saturated solution:
A solution that cannot dissolve more solute at a particular temperature is called a saturated solution.
Supersaturated solution:
A solution that contains more solute than is contained in the saturated solution is called a supersaturated solution.
(xv) Write short note on the following methods of prevention of corrosion:
a. Coating withy paints
b. Alloying
In progress
Chemistry 9 Solved Paper 2017
Section C
Attempt any TWO questions. All questions carry equal marks.
Q3. a. Write the postulates of Bohr’s Atomic Model. (Diagram is not necessary) (5)
Ans. Postulates of Bohr Atomic Model
1. The electron in an atom revolves around the nucleus in one of the circular orbits. Each orbit has fixed energy. So, each orbit is also called energy level.
2. The energy of the electron in an orbit is proportional to its distance from the nucleus. The farther the electron is from the nucleus, the more energy it has.
3. The electron revolves only in those orbits for which the angular momentum of the electron is an integral multiple of h/2π where his Plank’s constant (its value is 6.626×10-34 J.s).
4. Light is absorbed when an electron jumps to a higher energy orbit and emitted when an electron falls into a lower energy orbit. Electron present in a particular orbit does not radiate energy.
5. The energy of the light emitted is exactly equal to the difference between the energies of the orbits.
ΔE = E2 -E1
Where ΔE is the energy difference between any two orbits with energies E1 and E2.
Q3. b. Define Ionization energy. With the help of a suitable and clear reason, explain the trend of Ionization energy along the groups and periods. (1+4)
Ans. Ionization energy:
Definition:
“Ionization energy is defined as the minimum amount of energy required to remove the outermost electron from an isolated gaseous atom.”
M(g) + ionization energy → M+(g) + e–
Trend in the group:
From top to bottom in a group, ionization energy decreases.
Reason:
The shielding effect in atoms increases down the group which reserves weaker attraction of the nucleus and the valence electrons so they are easier to remove. Increase in atomic size down the group leads to a decrease in ionization energy.
Trend in the period:
From left to right in a period, ionization energy increases.
Reason:
The shielding effect remains constant but the nuclear charge increases in the period due to this force of attraction between the nucleus and the valence electron becomes stronger. So it is difficult to remove electrons from the Valence shell.
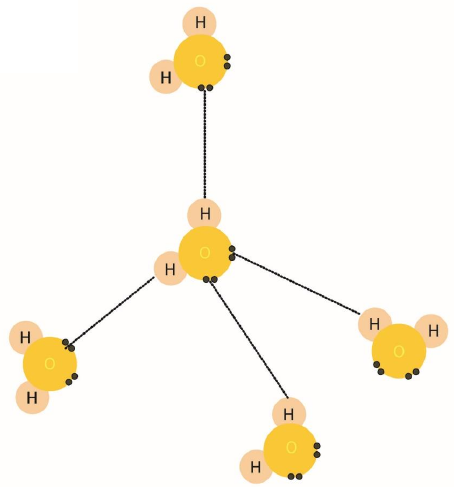
Q4. a. Define Hydrogen Bonding. Draw Hydrogen bonding in water. (1+1)
Ans. Hydrogen Bonding
The interaction of a highly electron-deficient hydrogen and lone pair on a nearby highly electronegative atom such as N, O, or F is called a hydrogen bond. This phenomenon is called hydrogen bonding.
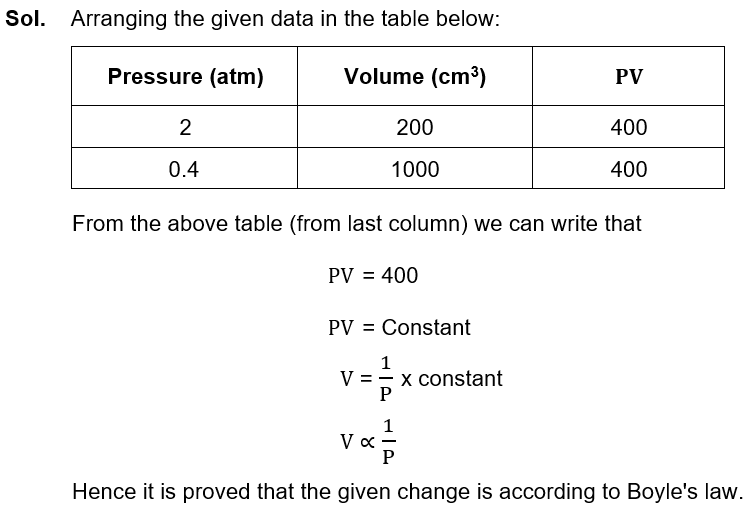
Q4. b. A gas was kept in 200cm3 container under 2 atm pressure. When it was shifted to 1000cm3 container its pressure reduced to 0.4 atm. Prove Boyle’s law with the help of this data. (3).
Q4. c. What is boiling point? Explain the effect of external pressure on the boiling point of water. (1+4)
Ans. Boiling Point
The temperature at which the vapour pressure of a liquid becomes equal to its external or atmospheric pressure is called the boiling point.
Effect of External Pressure on Boiling Point
By increasing external pressure, the boiling point is increased and vice versa.
For example, at sea level, the atmospheric pressure is 1 atm or 101.325 kPa so water boils at 100 °C.
At Mount Everest at a height of about 8850m above sea level, the atmospheric pressure becomes about 34 kPa. Therefore, at this low pressure, the water boils at 70 °C.
In Murree the vapour pressure of water becomes equal to atmospheric pressure at 98 °C therefore water boils at 98°C in Murree.
In a pressure cooker, there is a valve that exerts an external pressure of 2 atm. Because vapour pressure of water become 2 atm when the temperature reaches 120°C. So, water boils at 120°C in a pressure cooker.
Q5. a. Explain with suitable reason that why methanol is soluble but Gasoline is insoluble in water.ressure on the boiling point of water. (3)
Excluded from Syllabus
Q5. b. With the help of neat and clean diagram explain the manufacture of sodium metal from fused sodium chloride by Down’s cell. (7)
Excluded from Syllabus



I love this
Helpful
What a great upgraded content. I really appreciate you.
good info
Completed and good information
Very helpful webiste