Chemistry 9 Solved Paper 2019 Overseas Federal Board
Federal Board, Class 9 Chemistry Solved Past Paper 2019 Overseas is given below. The paper is solved according to the reduced syllabus for the annual examination 2021. Chemistry 9 Solved Paper 2019 Overseas is solved using the recommended textbook.
Chemistry 9 Solved Paper 2019 Overseas
Section A
Q1. MCQs
Chemistry 9 Solved Paper 2019 Overseas
Section B
Q2. Attempt any ELEVEN parts. The answer of each part should not exceed 3 to 4 lines.
(i) Differentiate ion and free radical. Give one example of each.
Excluded from Syllabus
(ii) Ozone is a gas that stops UV radiations. Fine the number of molecules in 3.5 moles of ozone gas.
Sol. Number of moles of ozone = 3.5 moles
Avogadro’s Number = NA = 6.022 x 1023
Number of molecules =?
Formula: Number of molecules = Number of moles x NA
Number of molecules = 3.5 x 6.022 x 1023
Number of molecules = 21.077 x 1023
Number of molecules = 2.1077 x 1024 molecules
(iii) Differentiate shells and subshells.
Ans.
| Shells | Sub-shells |
| 1. Shells or orbits are circular paths around the nucleus in which electrons revolve. | 1. A subshell is the area in which an electron moves within a shell. |
| 2. Each shell has fixed energy. It is also called energy level. | 2. It is called the sub-energy level. |
| 3. Names of shells are written in capital letters e.g., K, L etc. | 3. Names of sub-shells are written in small letters e.g., s, p etc. |
| 4. Number of electrons in any shell can be calculated by the formula 2n2, where n is the shell number. For example K shell: n=1 => 2(1)2= 2 electrons. M shell n=2 => 2(2)2= 8 electrons. N shell n=3 => 2(3)2= 18 electrons. And so on …. | 4. There are four sub-shells. Name and number of electrons in sub-shells are: s = 2 electrons p = 6 electrons d = 10 electrons f = 14 electrons |
(iv) Define:
a. Periodic law
b. Group of Periodic Table
c. Atomic size
Ans. a. Periodic Law:
This law states that “if the elements are arranged in the order of their increasing atomic numbers, their properties are repeated in a periodic manner”.
b. Group of Periodic Table: Each vertical column of elements in the periodic table is called a group or family. Elements with similar valance shell electronic configurations are placed in the same group. Each group is identified by a number and the letter A or B.
c. Atomic Size:
The size of an atom is the average distance between the nucleus of an atom and the outer electronic shell.
(v) Briefly explain the formation of ions of Aluminum and Fluorine.
Will be updated soon
(vi) How do the aqueous solution solutions of ionic compounds conduct electricity?
Excluded from Syllabus
(vii) Define Boyle’s law. Derive its mathematical expression.
Ans. Boyle’s Law
This law states that the volume of a given mass of a gas is inversely proportional to applied pressure at constant temperature.
Mathematically,
V ∝ 1 / P
V= 1 / P x constant
PV = constant
(viii) What is meant by vapour pressure? Why does the vapour pressure of a liquid increase by increasing temperature?
Ans. Vapour Pressure:
The pressure exerted by the vapours of a liquid in equilibrium with its liquid is called vapour pressure.
The vapour pressure of a liquid increases by increasing temperature because an increase in temperature increases the kinetic energy of its molecules. As a result, more of the molecules will have the minimum kinetic energy needed to escape the surface of the liquid.
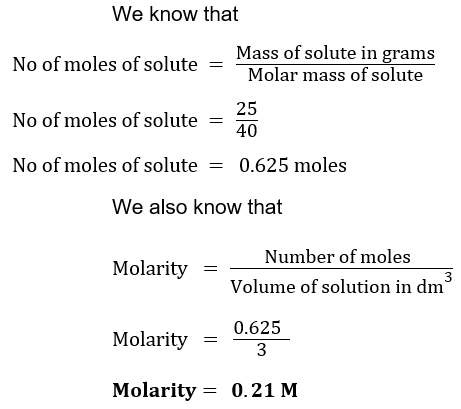
(ix) The Molar mass of sodium hydroxide is 40 g/mol. If you dissolve 25 g of it to make 3 dm3 solution, what will be its molarity?
Sol. Mass of NaOH = 25 g
Molar mass of NaOH = 40 g/mol
Volume of solution = 3 dm3
Molarity =?
(x) Two pairs of liquid are given. Which one is miscible and which one is not? State and give reason:
a. H2O and CCl4
b. H2O and Methanol
Excluded from Syllabus
(xi) Separate oxidizing and reducing agents in the following reactions:
a. H2S + Cl2 → 2HCl + S
b. Cu+2 (aq) + Zn(s) → Zn+2(aq) + Cu(s)
c. 2Mg + CO2 → 2MgO + C
Ans.
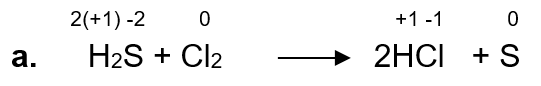
S is being oxidized because there is an increase in the oxidation state, so H2S is a reducing agent.
Cl is being reduced because there is a decrease in its oxidation state, so Cl2 is an oxidizing agent.
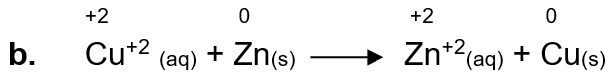
Zn is being oxidized because there is an increase in oxidation state, so Zn is a reducing agent.
Cu is being reduced because there is a decrease in its oxidation state, so Cu+2 is an oxidizing agent.
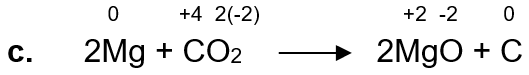
Mg is being oxidized because there is an increase in oxidation state, so Mg is a reducing agent.
C is being reduced because there is a decrease in its oxidation state, so CO2 is oxidizing agent.
(xii) Explain briefly the cathodic protection.
Ans. Cathodic protection:
1. Cathodic protection is the process in which the metal that is to be protected from corrosion is made the cathode.
2. It is connected to metal such as magnesium or aluminium which is more reactive than iron. The more active metal acts as an anode. It will oxidize and save iron from corrosion.
Importance:
Cathodic protection is employed to prevent iron and steel structures such as pipes, tanks, oil rigs, etc which are found in the moisture, underground, and marine environment.
(xiii) a. Why are alkali metals kept in kerosene oil?
Ans: Alkali metals are highly reactive because they have only one electron in their valence shell which can easily be lost and metal get oxidized. In presence of water, they react to form highly flammable gas Hydrogen, so they are kept in kerosene.
(xiii) b. Complete the following reactions:
(i) 2Na + 2H2O →
(ii) Mg + H2O →
Ans. (i) 2Na + 2H2O → 2NaOH + H2
(ii) Mg + H2O → MgO + H2
(xiv) Write any three applications of Platinum.
Ans: 1. Platinum is widely used as a catalyst for many types of industrial processes. For example, 100% pure sulfuric acid is prepared by contact process in which platinum is used as a catalyst.
2. It is used as an electrode as a part of hydrogen electrode in cells.
3. Automobile exhaust is a major source of air pollution. Therefore, most new cars are equipped with catalytic converters. It contains platinum which catalyzes the complete combustion of carbon monoxide and hydrocarbon.
(xv) Write the electronic configuration of the following by using 1s, 2s…..formula: (a) Ar (b) Si (c) N
Ans. (a) Ar = 1s2, 2s2, 2p6, 3s2, 3p6, 4s1
(b) Si = 1s2, 2s2, 2p6, 3s2, 3p2
(c) N = 1s2, 2s2, 2p3
Chemistry 9 Solved Paper 2019 Overseas
Section C
Attempt any TWO questions. All questions carry equal marks.
Q3. a. Write the postulates of Bohr atomic model. (5)
Ans. Postulates of Bohr Atomic Model
1. The electron in an atom revolves around the nucleus in one of the circular orbits. Each orbit has fixed energy. So, each orbit is also called energy level.
2. The energy of the electron in an orbit is proportional to its distance from the nucleus. The farther the electron is from the nucleus, the more energy it has.
3. The electron revolves only in those orbits for which the angular momentum of the electron is an integral multiple of h/2π where his Plank’s constant (its value is 6.626×10-34 J.s).
4. Light is absorbed when an electron jumps to a higher energy orbit and emitted when an electron falls into a lower energy orbit. Electron present in a particular orbit does not radiate energy.
5. The energy of the light emitted is exactly equal to the difference between the energies of the orbits.
ΔE = E2 -E1
Where ΔE is the energy difference between any two orbits with energies E1 and E2.
Q3. b. What is meant by shielding effect? Also write and give reason for its trends in periodic table. (1+4)
Ans. Shielding Effect:
Definition:
The reduction in force of attraction between the nucleus and the valence electrons by the electrons present in the inner subshells is called the shielding effect.
Trend in the group:
From top to bottom in a group shielding effect increases.
Reason:
As the number of the electronic shell increases so the number of electrons in the inner shell also increases as a result shielding effect increases.
Trend in period:
From left to right in a period shielding effect also remains constant.
Reason:
As the number of electrons in the inner shells remains constant, as a result, the shielding effect remains constant.
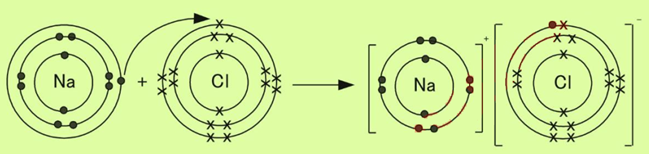
Q4. a. What is ionic bond? Explain the formation of ionic bond in sodium chloride. (2+3)
Ans. Ionic Bond
The forces of attraction that bind oppositely charged ions are called ionic bonds.
Formation of Sodium Chloride
The ionic bond is formed between sodium and chlorine. Sodium (Na) is metal and (Chlorine) Cl is non-metal.
Metal atom tends to lose electrons and non-metal atom tends to gain electrons to acquire electronic configuration of the nearest noble gas. Since the Na atom has one electron in the outermost shell, it losses one electron to form an ion. Since the Cl atom has seven electrons in the outermost shell, it needs one electron to complete the octet. So, it gains one electron to form an ion. For every ion, we need one ion.
Q4. b. Explain the effect of external pressure on boiling point. (5)
Effect of External Pressure on Boiling Point
By increasing external pressure, the boiling point is increased and vice versa.
For example, at sea level, the atmospheric pressure is 1 atm or 101.325 kPa so water boils at 100 °C.
At Mount Everest at a height of about 8850m above sea level, the atmospheric pressure becomes about 34 kPa. Therefore, at this low pressure, the water boils at 70 °C.
In Murree the vapour pressure of water becomes equal to atmospheric pressure at 98 °C therefore water boils at 98°C in Murree.
In a pressure cooker, there is a valve that exerts an external pressure of 2 atm. Because vapour pressure of water becomes 2 atm when the temperature reaches 120°C. So, water boils at 120°C in a pressure cooker.
Q5. a. Define solution, suspension and colloid. (3)
Ans. Solution
A solution is a homogeneous mixture in which the particles are individual molecules or ions are distributed evenly throughout the fluid.
Suspension
A suspension is a heterogeneous mixture containing particles large enough to be seen with the naked eye and clearly distinct from the surrounding fluid.
Colloid
A colloid is a heterogeneous mixture of tiny particles of a substance dispersed through a medium.
Q5. b. How is sodium manufactured from fused sodium chloride? Support your answer with equations and diagram.(3+2+2)
Excluded from Syllabus