Class 10 Chemistry 2019 Solved Past Paper Federal Board
Federal Board, Class 10 Chemistry 2019 Past Papers Local is solved in this post. For more solved past papers visit our past papers page.
Class 10 Chemistry 2019 Solved Paper
Section A
Q1. MCQs
Class 10 Chemistry 2019 Solved Paper
Section B
Q2.
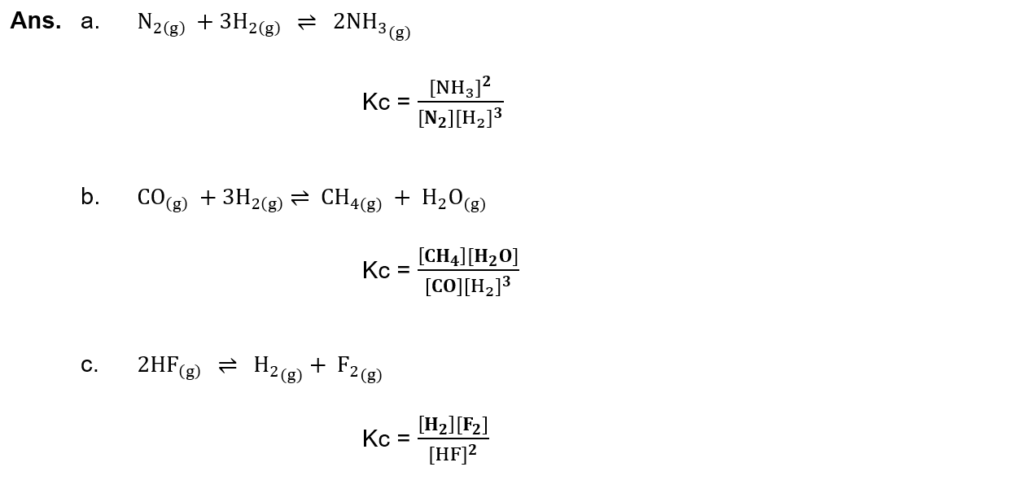
(i) Write the equilibrium constant expressions for the following chemical reactions:
(ii) How does milk of magnesia neutralize excess stomach acid?
Ans. Since milk of magnesia Mg(OH)2 is a base, it neutralizes excess stomach acid (HCl) by carrying out a neutralization reaction with it. The reaction is given below:
Mg(OH)2 + 2HCl → MgCl2 + 2H2O
(iii) Differentiate between an alkane and an alkyl radical with the help of examples.
Ans.
| Alkane | Alkyl Radical |
| Alkane is a saturated hydrocarbon containing only single bonds. | Alkyl radical is a group of atoms obtained by removing one hydrogen atom from an alkane. |
| Alkane has no free valency. | Alkyl radical has one free valency. |
| Its general formula is CnH2n+2 | Its general formula is CnH2n+1 |
(iv) What is a substitution reaction? How halogen atom substitutes hydrogen atom of an alkane?
Ans. Substitution Reaction
A substitution reaction (also known as a single displacement reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group.
Example of a substitution reaction is a reaction of halogen with alkane (also known as halogenation).
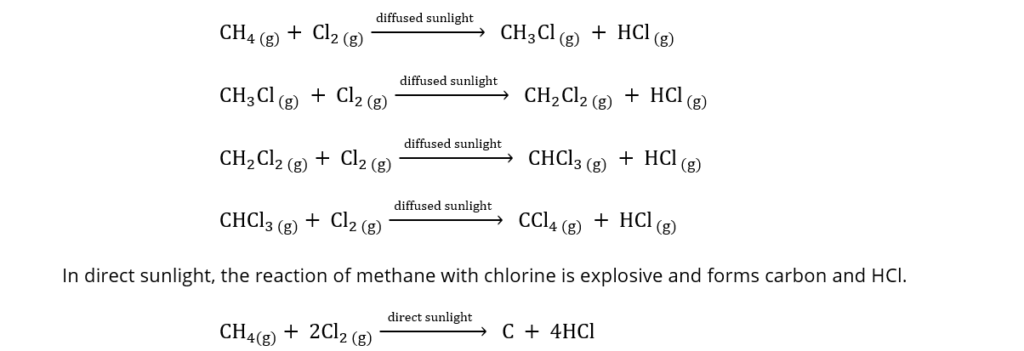
For example, the reaction of methane and chlorine in diffused sunlight occurs as follows:
(v) Differentiate fat soluble vitamins from water soluble vitamins with suitable examples.
Ans.
| Fat-soluble vitamins | Water-soluble vitamins |
| A vitamin that dissolves in fat is called a fat-soluble vitamin. | A vitamin that dissolves in water is called a water-soluble vitamin. |
| They may be harmful when taken in excess. | They are not toxic even if taken in excess. |
| They can’t be excreted by urination. | They can be excreted by urination. |
| For example, vitamin A, D, E, and K | For example, vitamin B complex and C |
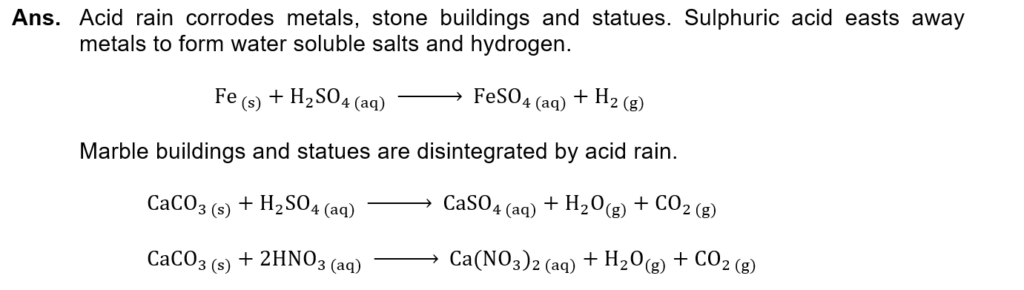
(vi) What is the effect of acid rain on iron and marble? Give balanced chemical reactions.
(vii) How do ultraviolet radiations break chlorofluorocarbons?
Ans. Ultraviolet radiations break chlorofluorocarbons (CFCs) molecules and produce chlorine free radicals. The reaction is given below:
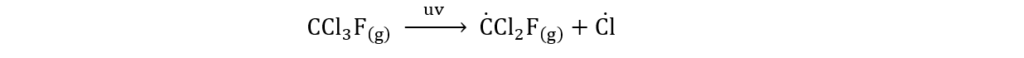
(viii) Which properties of water make it universal solvent?
Excluded from Syllabus for Annual Exams 2021
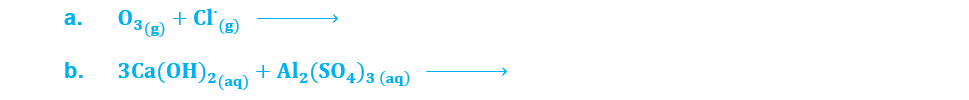
(ix) Complete the following chemical reactions:
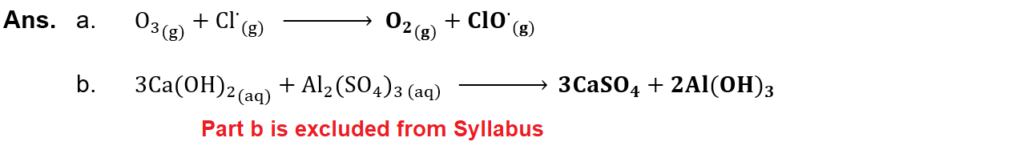
(x) What is glycogen? What is its role in animal body?
Ans. Glycogen is a polysaccharide of glucose that serves as a long-term energy reservoir in animal bodies.
Glucose is stored in animal muscles and liver cells in the form of glycogen. It can be converted back to glucose when energy is needed by the animal body.
(xi) What raw materials are used to manufacture sodium carbonate?
Ans. Following raw materials are used to manufacture sodium carbonate:
(a) Ammonia
(b) Brine (concentrated sodium chloride solution)
(c) Limestone as a source of carbon dioxide and slaked lime, Ca(OH)2
(xii) Why is cellulose part of human diet?
Ans. Cellulose is part of the human diet because:
- It helps the muscles of our intestines to move food efficiently through the digestive track.
- It absorbs and carries away toxic chemicals in food that would otherwise harm us.
- It also helps in lowering cholesterol and regulates blood pressure.
(xiii) Identify the functional groups in a typical amino acid. Draw a peptide linkage between two amino acids.
Excluded from Syllabus for Annual Exams 2021
(xiv) What is slaked lime? How is it produced?
Ans. Calcium Hydroxide Ca(OH)2 is also known as Slaked Lime. Slaked Lime is produced in a kiln by mixing equal amounts of lime (CaO) and water H2O. The chemical reaction is as under:
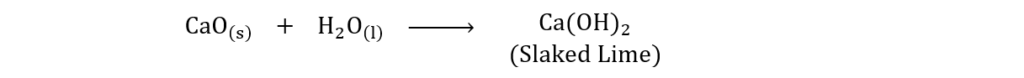
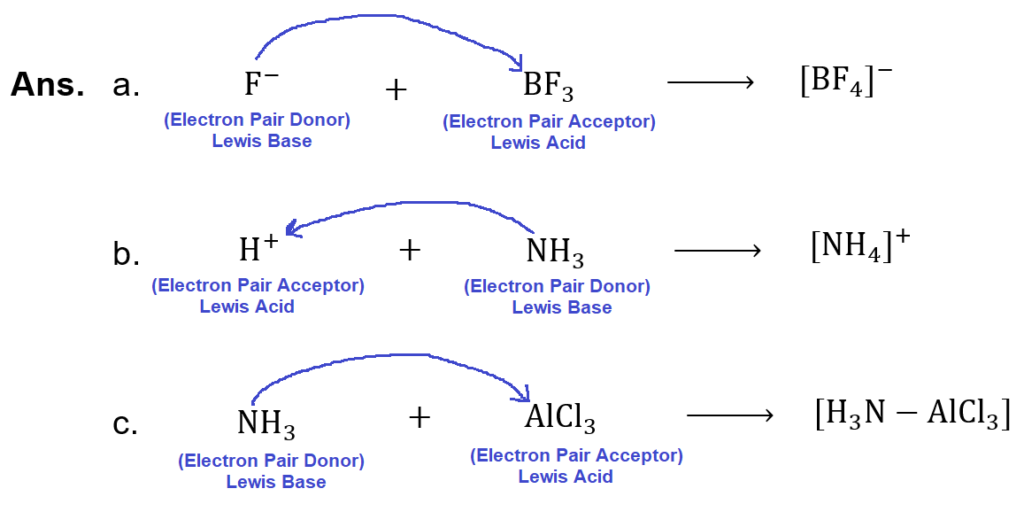
(xv) Identify Lewis acid and Lewis base in the following reactions:

Section C
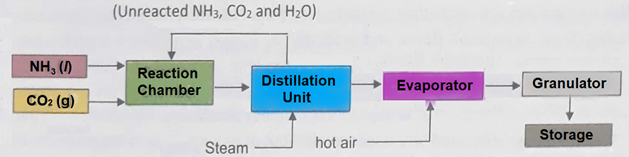
Q3.a. Explain the manufacturing of Urea with the help of chemical reactions. Also draw flow sheet for urea manufacturing. (4+2)
Ans. Manufacturing of urea consists of the following steps:
(a) Reaction between NH3 and CO2 to form ammonium carbamate.
2NH3 + CO2 → NH2COONH4
(b) Distillation of ammonium carbamate
NH2COONH4 → NH2CONH2 + H2O
(c) Evaporation of liquid Urea and its granulation.
The Urea solution is concentrated in vacuum evaporators, which are then rapidly cooled and sent to the prilling tower. Urea prills thus produced are packed and then marketed.
Flowsheet Diagram
Q3.b. Write four disadvantages of hard water. (4)
Excluded from Syllabus for Annual Exams 2021
Q4.a. Describe structure and function of DNA. (4+2)
Ans. Structure of DNA
DNA exists in the form of two strands twisted around each other in a spiral formation called a double helix. Each chain or strand is made up of a deoxyribose sugar, phosphate unit, and nitrogen base. The strands are held together by hydrogen bonds. The order of the base pairs in a strand is a code that stores information that is used to produce proteins. The key to the ability of DNA to store genetic information and to pass it on from generation to generation is its double-stranded structure.
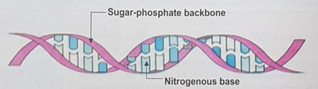
Function of DNA
The function of DNA is to store and transmit all the genetic information needed to build an organism. For instance, in human beings, the single fertilized egg cell carries the information for making legs, hands, head, liver, heart, kidneys, etc. DNA is found primarily in the cell nucleus.
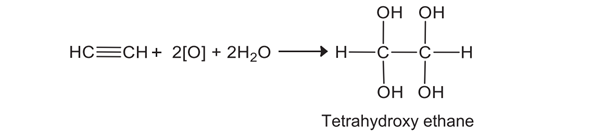
Q4.b. Give the reaction of Ethyne with strong alkaline solution of KMnO4. (4)
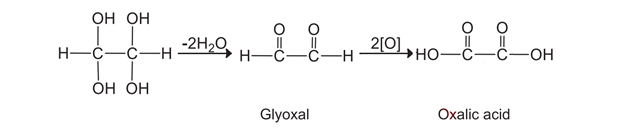
Ans. Alkynes do not react with a dilute alkaline aqueous solution of KMnO4. However, they are oxidized by a strongly alkaline solution of KMnO4 to give oxalic acid. The first four hydroxyl groups are added across the triple bond.
4KMnO4 + 4KOH → 4K2MnO4 + 4[O] + 2H2O
Tetrahydroxy ethane is an unstable compound; it loses two water molecules to form glyoxal which finally oxidizes to oxalic acid.
Q5.a. Define Salt. Discuss various methods for making salt. (1+5)
Ans. Salt
Salt is an ionic compound formed when a replaceable hydrogen atom in an acid is replaced by a metal atom.
Methods of making Salts
There are five methods for making salts:
1. Acid + Base → Salt + Water
HCl + NaOH → NaCl + H2O
2. Acid + Metal Oxide → Salt + Water
H2SO4 + CuO → CuSO4 + H2O
3. Acid + Metal → Salt + Hydrogen
2HCl + Mg → MgCl2 + H2
4. Acid + Metal Carbonate → Salt + Carbon dioxide + Water
HCl + CaCO3 → CaCl2 + CO2 + H2O
5. Salt + Salt → Salt + Salt
AgNO3 + NaCl → AgCl + NaNO3
Q5.b. What is self-ionization of water? Explain self-ionization of water with equation. (1+3)
Excluded from Syllabus for Annual Exams 2021