Class 10 Chemistry Solved Past Paper 2020 Federal Board
Federal Board Class 10 Chemistry Solved Past Paper 2020 Federal Board. Paper is solved for helping students. For more solved papers visit Class 10 Solved Past Papers page.
Class 10 Chemistry Solved Past Paper 2020
Section A
Q1. MCQs
Not Available
Class 10 Chemistry Solved Past Paper 2020
Section B
Q2.
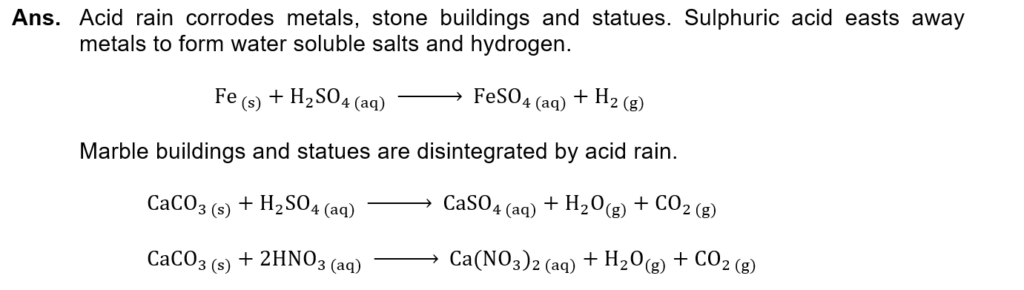
(i) What is the effect of acid rain on iron and marble? Describe by balanced chemical equations.
(ii) Write down the industrial uses of Ethyne.
Ans. Industrial Uses of Ethyne:
1. In oxy-acetylene torch for welding and cutting metals.
2. For ripening of fruits.
3. For the manufacture of polyvinyl acetate (PVA), polyvinyl chloride (PVC), polyvinyl ethers, and rubber.
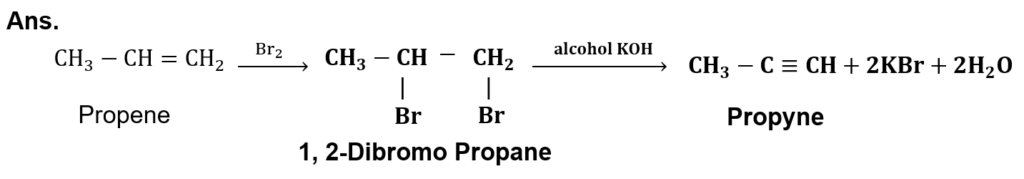
(iii) Identify the products A and B in the following reactions:

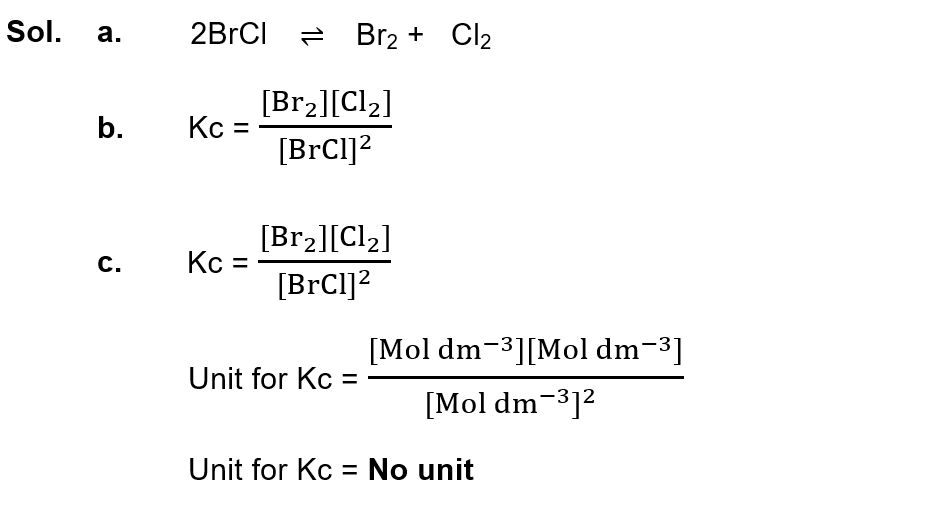
(iv) Bromine Chloride (BrCl) decomposes to form bromine and chlorine. For this reaction write:
a. Chemical equation
b. Kc expression
c. Units for Kc
(v) What raw materials are used to manufacture sodium carbonate?
Ans. Following raw materials are used to manufacture sodium carbonate:
(a) Ammonia
(b) Brine (concentrated sodium chloride solution)
(c) Limestone as a source of carbon dioxide and slaked lime, Ca(OH)2
(vi) Dibenzothiophene (C12H6S) is a common sulphur containing compound of coal. Can it cause acid rain? If yes, how?
Ans. Dibenzothiophene (C12H6S) is a sulfur-containing compound of coal. On burning, it produces sulfur dioxide. In the air sulfur dioxide is converted into sulfur trioxide which is responsible for acid rain.
(vii) Write down the formula of amino acid. Show peptide linkage between two amino acids units.
Excluded from Syllabus for Annual Exams 2021
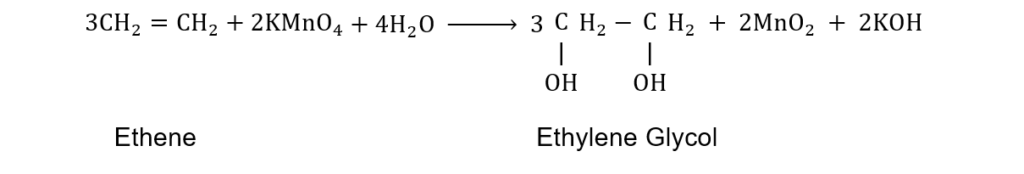
(viii) What do you understand by Baeyer’s test? Write chemical equation showing reaction of KMnO4 with alkene?
Ans. When an alkene is treated with a dilute alkaline aqueous solution of KMnO4 (1%) addition of two hydroxyl groups across the double bond. The pink colour of the KMnO4 solution is discharged during the reaction. This reaction is used as a test for the presence of an alkene and is known as Baeyer’s test.
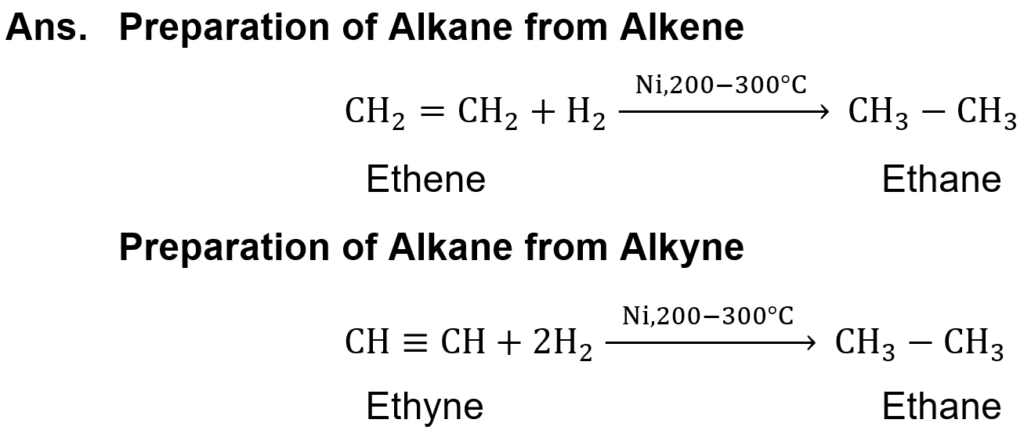
(ix) Write chemical equations to show the preparation of an alkane from both alkene and alkyne.
(x) Identify the functional group in following compounds and write names of these groups. Also encircle the functional group:
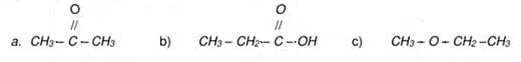
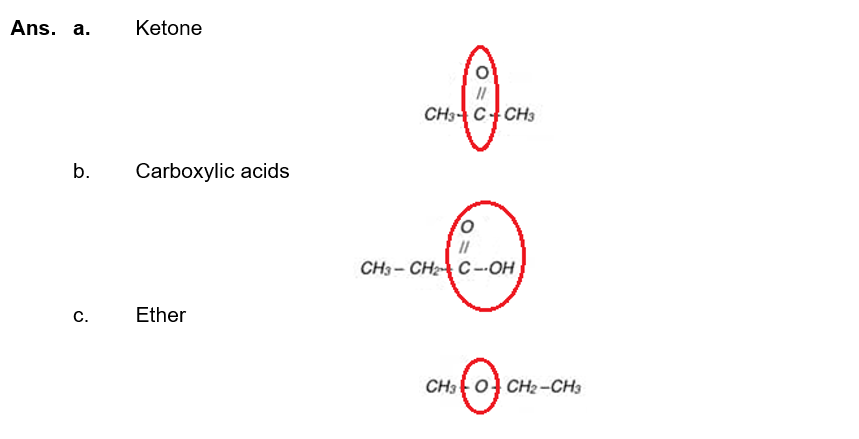
(xi) State the conditions for equilibrium.
Ans. Conditions of Equilibrium
1. Concentration of the reactant or product remains unchanged.
2. Temperature of the system remains constant.
3. Pressure or volume of the system remains constant.
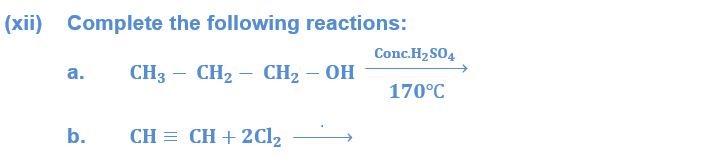
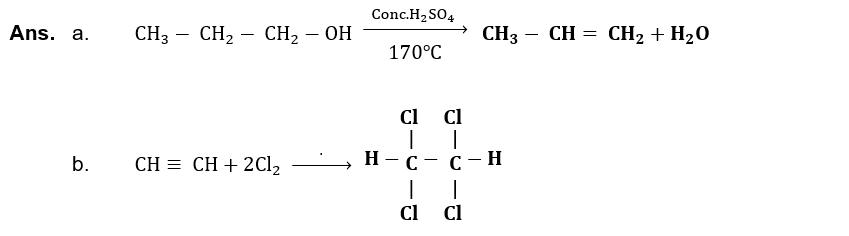
(xiii) Write down structural formula of glucose and fructose, also identify functional groups in both of them.
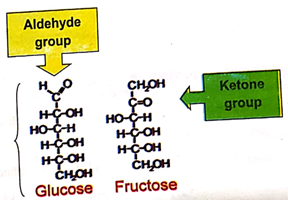
(xiv) What is the function of DNA?
Ans. The function of DNA is to store and transmit all the genetic information needed to build an organism. For instance, in human beings, the single fertilized egg cell carries the information for making legs, hands, head, liver, heart, kidneys etc. DNA is found primarily in the cell nucleus.
(xv) How is the permanent hardness of water removed by ion exchange resins?
Ans. By ion Exchange Resins
The hard water is passed through a container filled with a suitable resin containing sodium ions. Zeolite is one of the natural ion exchangers. Chemically it is sodium aluminum silicate. It is usually written as Na2Z. The Ca+2 or Mg+2 ions causing the hardness are exchanged with Na+ ions in the resin.
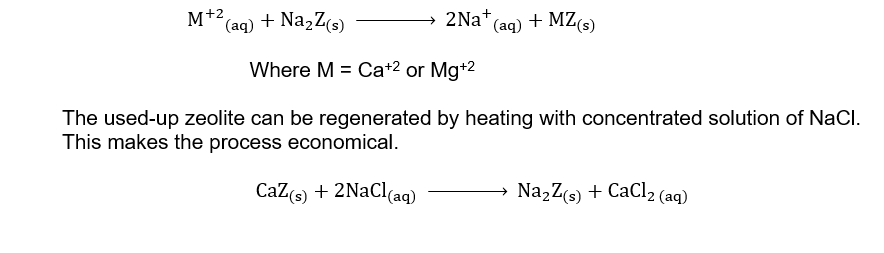
Class 10 Chemistry Solved Past Paper 2020
Section C
Q3.a. Describe different stages of Raw water treatment.
Excluded from Syllabus for Annual Exams 2021
Q3.b. Write the methods to remove temporary hardness of water.
Ans. Methods to remove temporary hardness
i) By Boiling
The hardness of water can be removed simply by boiling. During boiling the soluble calcium and magnesium hydrogen carbonates are decomposed forming insoluble carbonates. Since Ca+2 and Mg+2 ions are removed as insoluble carbonates, water becomes soft.
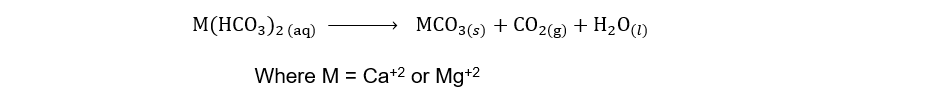
Unfortunately, this method is too expensive to remove the temporary hardness of the water on a large scale.
ii) By adding slaked lime (Clark’s method)
Temporary hardness in water on a large scale can be removed by adding an estimated amount of slaked lime in it. The slaked lime reacts with the hydrogen carbonates to form insoluble carbonates.
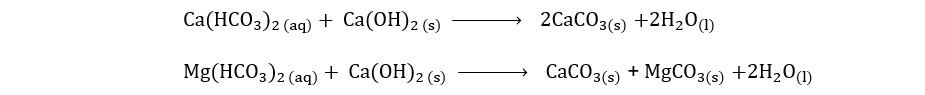
Q4.a. Write different methods for making salts.
Ans. There are five methods for making salts:
1. Acid + Base → Salt + Water
HCl + NaOH → NaCl + H2O
2. Acid + Metal Oxide → Salt + Water
H2SO4 + CuO → CuSO4 + H2O
3. Acid + Metal → Salt + Hydrogen
2HCl + Mg → MgCl2 + H2
4. Acid + Metal Carbonate → Salt + Carbon dioxide + Water
HCl + CaCO3 → CaCl2 + CO2 + H2O
5. Salt + Salt → Salt + Salt
AgNO3 + NaCl → AgCl + NaNO3
Q4b. Describe different types of vitamins.
Ans. Types of Vitamins
There are two types of vitamins:
(a) Fat-soluble vitamins
A vitamin that dissolves in fat is called a fat-soluble vitamin. For example, vitamin A, D, E, and K. Taking the excess amount of fat-soluble vitamins may be harmful. For instance, a large excess of vitamin, A can cause irritability, dry skin, and feeling of pressure inside the head. Too much vitamin D can cause pain in bones, hard deposits in joints and kidneys, and weight loss.
(b) Water-soluble vitamins
A vitamin that dissolves in water is called a water-soluble vitamin. For example, vitamins B (complex) and C. Our body has limited capacity to store these vitamins. If taken in excess, these are readily excreted from the body. Water-soluble vitamins are not toxic even if taken in excess.
Q5.a. Explain the temperature variations in thermosphere (maximum and minimum). List the components of thermosphere.
Ans. Temperature Variations in Thermosphere
The outermost layer of the atmosphere is the thermosphere. It extends from 80 km above Earth’s surface outward into space. The temperature in the thermosphere varies from -93 ˚C to 1800 ˚C.
Components of Thermosphere
The thermosphere is divided into two main layers. The lower layer called the ionosphere extends from 80 km to 400 km above the surface of Earth. The outer layer of the thermosphere is the exosphere. It extends from 400 km to thousands of kilometers from Earth’s surface.
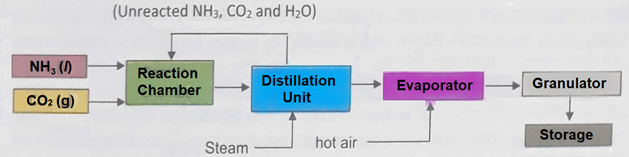
Q5.b. Explain the manufacturing of urea with the help of chemical reactions. Also draw flow sheet for urea manufacturing.
Ans. Manufacturing of urea consists of the following steps:
(a) Reaction between NH3 and CO2 to form ammonium carbamate.
2NH3 + CO2 → NH2COONH4
(b) Distillation of ammonium carbamate.
NH2COONH4 → NH2CONH2 + H2O
(c) Evaporation of liquid Urea and its granulation.
The Urea solution is concentrated in vacuum evaporators, which is then rapidly cooled and sent to the prilling tower. Urea prills thus produced are packed and then marketed.
Flowsheet Diagram: