Class 10 Chemistry 2018 Solved Past Paper Federal Board
Federal Board, Class 10 Chemistry 2018 Past Papers Local is solved in this post. For more solved past papers visit our past papers page.
Class 10 Chemistry 2018 Solved Paper
Section A
Q1. MCQs
Class 10 Chemistry 2018 Solved Paper
Section B
Q2.
(i) What are the conditions for equilibrium to be continued?
Ans. Conditions of Equilibrium
1. Concentration of the reactant or product remains unchanged.
2. Temperature of the system remains constant.
3. Pressure or volume of the system remains constant.
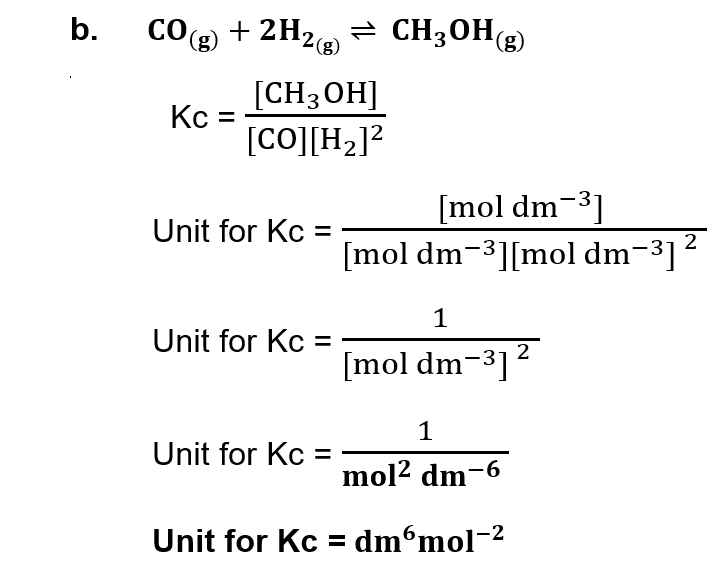
(ii) Derive unit of Kc for the following equilibrium reactions:


(iii) a. Define Arrhenius acid and write an example.
Ans. Arrhenius Concept of Acid
An acid is a substance that ionizes in water to produce H+ ions.
Example:

(iii) b. Define Lewis acid and write an example.
Ans: Lewis Concept of Acid
A Lewis acid is a substance that can accept a pair of electrons to form a coordinate covalent bond.
Example:
In the following reaction, the Hydrogen atom in HCl is an electron pair acceptor, so HCl is an acid.


Excluded from Syllabus for Annual Exams 2021
(v) Define saturated and unsaturated hydrocarbons and write one example of each.
Ans. Saturated Hydrocarbons
Hydrocarbons whose carbon-carbon bonds are all single are called saturated hydrocarbons. Saturated hydrocarbons are also called alkanes.
Example: Methane CH4
Unsaturated Hydrocarbons
Hydrocarbons containing carbon-carbon multiple bonds are called unsaturated hydrocarbons.
Example: Ethene C2H6
(vi) Define open chain and closed chain organic compounds and write one example of each.
Ans. Open Chain Organic Compounds
Organic compounds which contain an open chain of carbon atoms are called open-chain or alicyclic compounds.
Example: Propane CH3 – CH2 – CH3
Closed Chain Organic Compounds
Organic compounds which contain rings of atoms are called closed chain or cyclic compounds.
Example:

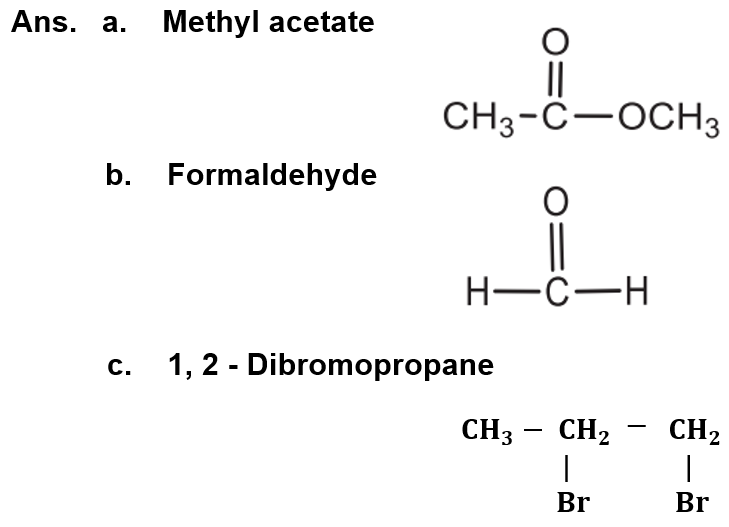
(vii) Write structural formula of:
a. Methyl acetate
b. Formaldehyde
c. 1, 2 – Dibromopropane
(viii) Complete the following equations:
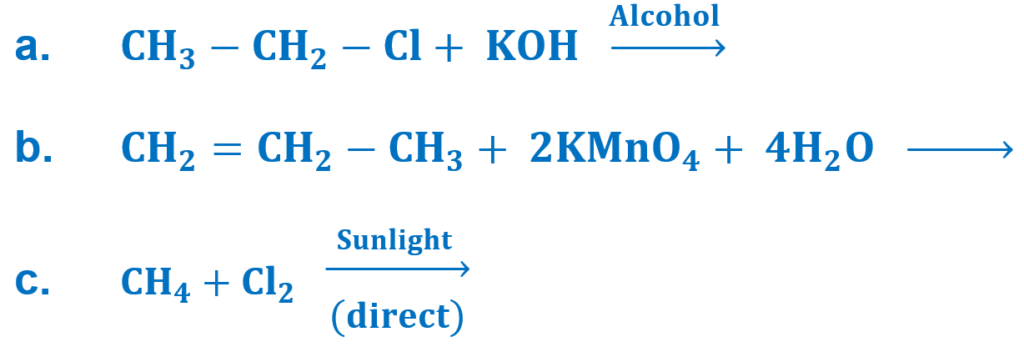

(ix) How can you convert?
a. Ethanol into Ethene
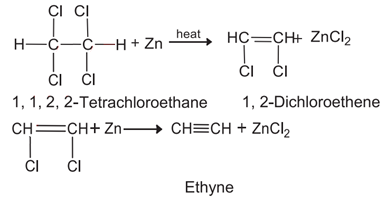
b. 1, 1, 2, 2 – Tetrachloroethane into Ethyne.
Ans. a. Ethanol into Ethene
Ethanol can be converted into Ethene on passing its vapours over heated alumina.

b. 1, 1, 2, 2 – Tetrachloroethane into Ethyne
(x) What are polysaccharides? Write their two physical properties.
Ans. Polysaccharides
Carbohydrates which upon hydrolysis form 10 to 1000 units of simple sugars are called polysaccharides. Starch and cellulose are polysaccharides.
Physical Properties
1. They are amorphous solids.
2. They are tasteless and insoluble in water.
(xi) What are pollutants? Write any two effects of sulphur dioxide on human beings.
Ans. Pollutants
Anything that is in the air, water or soil which has a harmful effect on some part of the environment is called a pollutant.
Effects of Sulphur Dioxide on Human Beings
1. It is readily absorbed in the respiratory system.
2. Being a powerful irritant, it aggravates the symptoms of people who suffer from asthma, bronchitis, emphysema and other lung diseases.
(xii) What is meant by acid rain? Write its two effects.
Ans. Acid rain is defined as rain having pH less than 5.6.
Effects of Acid Rain (Write any two)
1. Acid rain (Sulphuric acid) easts away metals to form water soluble salts and hydrogen.
2. Marble buildings and statues are disintegrated by acid rain.
3. Acid rain kills fish and destroys trees.
(xiii) Describe coagulation step in raw water treatment.
Excluded from Syllabus for Annual Exams 2021
(xiv) Write short note on: a. Dysentery b. Hepatitis
Ans. Dysentery
Dysentery is also an intestinal disease. It is caused by a parasite, Entamoeba. This infection is transmitted by faecal contamination of water or food by the encysted organisms. Patients have mild to severe abdominal cramps, diarrhea, chocolate coloured stool with mucous and some, with blood.
Hepatitis
Hepatitis is an acute inflammation of the liver. It is caused by viruses, and classified as hepatitis A, B, C, D and E. Hepatitis A and E spreads through polluted water.
(xv) How is copper ore concentrated by Froth flotation process?
The answer will be updated soon
Class 10 Chemistry 2018 Solved Paper
Section C
Q3.a. What is salt? Write five methods with equations for preparation of salts. (1+5)
Ans. Salt
Salt is an ionic compound formed when a replaceable hydrogen atom in an acid is replaced by a metal atom.
Methods of making Salts
There are five methods for making salts:
1. Acid + Base → Salt + Water
HCl + NaOH → NaCl + H2O
2. Acid + Metal Oxide → Salt + Water
H2SO4 + CuO → CuSO4 + H2O
3. Acid + Metal → Salt + Hydrogen
2HCl + Mg → MgCl2 + H2
4. Acid + Metal Carbonate → Salt + Carbon dioxide + Water
HCl + CaCO3 → CaCl2 + CO2 + H2O
5. Salt + Salt → Salt + Salt
AgNO3 + NaCl → AgCl + NaNO3
Q4.b. How are alkanes prepared by hydrogenation of alkenes and reduction of alkyl halides? Describe and support your answer with equations. (2+2)
Ans. By Hydrogenation of alkenes
The addition of hydrogen molecules across carbon-carbon multiple-bond is called hydrogenation. Hydrogenation takes place in presence of finely divided nickel at 200-300° C and high pressure. Hydrogenation can also be done in presence of Pt or Pd at room temperature.

By the reduction of alkyl halides
When an alkyl halide is treated with Zn in presence of aqueous acid, an alkane is produced. Usually, the aqueous solution of HCI or CH3COOH is used.

Zn reacts with aqueous acid to liberate atomic hydrogen called nascent hydrogen. Nascent hydrogen reduces alkyl halide. The addition of nascent hydrogen is called reduction.
Q4.a. Write equation for formation of a dipeptide. Also write any four uses of proteins.
Excluded from Syllabus for Annual Exams 2021
Q4.b. What is global warming? Write its three effects on earth. (1+3)
Ans. The warming of the atmosphere which is due to our influence on the greenhouse effect is known as global warming.
Effects of Global warming on Earth
1. Temperature of the earth will gradually increase.
2. Polar ice may melt and cause a significant increase in sea levels.
3. The atmosphere becomes hotter.
Q5.a. Write a method for removal of permanent hardness of water. (03)
Ans. Method to remove permanent hardness
By adding washing soda
On the large scale permanent hardness in water can be removed by adding washing soda (Na2CO3.10H2O). Ca+2 and Mg+2 ions are removed as their insoluble carbonates.

Q5.b. Write the main steps of Solvay’s process supported with chemical equations. (07)
Ans. Solvay Process
Sodium carbonate (Na2CO3) or soda ash is an important industrial chemical. It is manufactured in a continuous process known as the Solvay process.
Main Steps of Solvay Process
The Solvay process consists of the following steps:
i) Preparation of ammonical brine:
Ammonical brine is prepared by dissolving ammonia gas in brine. Ammonical brine is fed into the carbonating tower.
ii) Carbonation:
In the carbonating tower, carbon dioxide is passed through ammonical brine. Following reaction takes place in it.
CO2(g) + NH3(g) + H2O(l) → NH4HCO3(aq)
NH4HCO3(aq) + NaCl(aq) → NaHCO3(s) + NH4Cl(aq)
In the lower compartments of carbonating tower, the temperature of the mixture is lowered to 15°C. At this temperature, NaHCO3 precipitates out.
iii) Filtration
Precipitates of NaHCO3 are separated from the milky solution by filtration. It is used as baking soda.
iv) CalcinationSodium hydrogen carbonate is heated to get sodium carbonate.
2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
Carbon dioxide released is re-cycled in the process.
v) Preparation of carbon dioxide and slaked lime
Carbon dioxide is produced by heating limestone in a kiln.
CaCO3(l) → CaO(s) + CO2(g)
Slaked lime is pumped to the ammonia recovery tower.
vi) Recovery of ammonia
A solution containing ammonium chloride produced in the carbonation tower is heated with slaked lime.
2NH4Cl(aq) + Ca (OH)2(aq) → 2NH3(g) + CaCl2(aq) + 2H2O(l)
Almost all the ammonia is recovered in this process. It is reused in the process.